Indole derivative and preparation method thereof
A technology of indole derivatives and compounds, applied in the field of indole derivatives and their preparation, can solve the problems of complex structure, heavy metal pollution, and inability to recycle, and achieve high reference value, mild conditions, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] A kind of preparation method of indole derivative provided by the present invention comprises the following steps:
[0029] Reacting a mixture of one of the compounds represented by formula I, an additive and a solvent under conditions of nitrogen or argon and visible light to obtain an indole derivative;
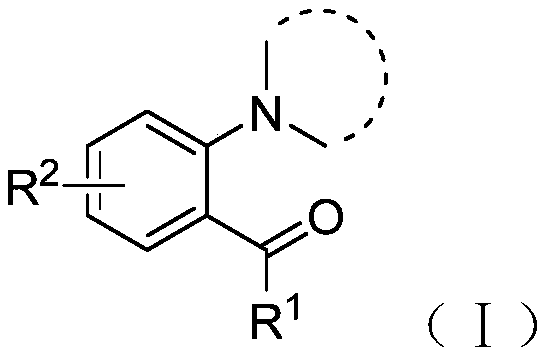
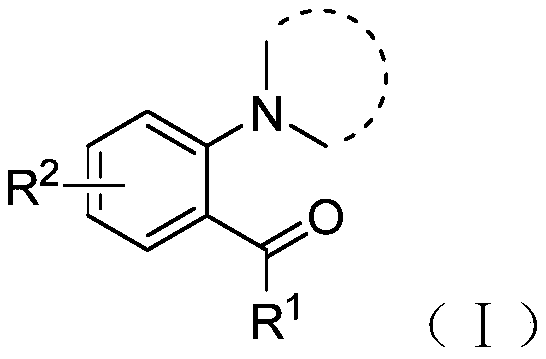
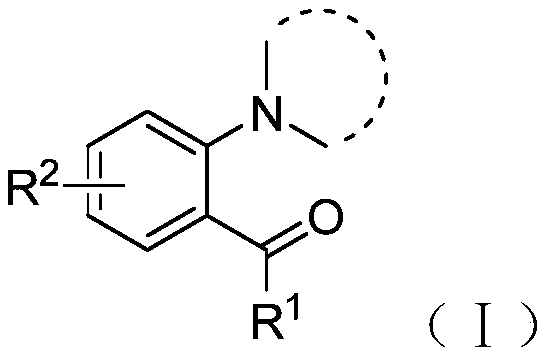
[0030] The structural formula of the compound shown in the formula I is as follows:
[0031]
[0032] Among them, R 1 One selected from hydrogen, methyl, ethyl, cyclopropyl, cyclohexyl, phenyl, 4-methylphenyl, 4-chlorophenyl, 3-chlorophenyl or 3-methoxyphenyl ;
[0033] R 2 One selected from hydrogen, chlorine, bromine, methoxy or cyano.
[0034] The synthetic route is as follows:
[0035]
[0036] In the preparation method of indole derivatives provided by the present invention, in a nitrogen or argon atmosphere, visible light irradiation can excite the compound shown in formula I to undergo homolysis of the carbon-oxygen bond, and then [1,6]-H migration ...
Embodiment 1
[0048]
[0049] In a 10mL quartz test tube, add p-toluenesulfonic acid (4.0mg, 0.02mmol), 2-pyrrolidinylbenzaldehyde (35.0mg, 0.2mmol), N-methylpyrrolidone (2mL), argon protection, blue LED Under lamp irradiation, stir at room temperature for 24 hours. After the reaction was completed, N-methylpyrrolidone was removed by washing with water, and the product (14.1 mg, yield 47%) was obtained by silica gel column chromatography using a mixed solvent of petroleum ether and ethyl acetate as the eluent.
[0050] 1 H NMR (400MHz, CDCl3) δ7.57(d, J=7.8Hz, 1H), 7.27(d, J=8.0Hz, 1H), 7.14(t, J=7.0Hz, 1H), 7.08(t, J =7.0Hz,1H),6.19(s,1H),4.09(t,J=7.0Hz,2H),3.05(t,J=7.4Hz,2H),2.71–2.55(m,2H).
[0051] 13 C NMR (100MHz, CDCl 3 )δ144.61, 133.30, 132.70, 120.33, 120.14, 119.13, 109.41, 92.31, 43.62, 27.88, 24.32.
Embodiment 2
[0053]
[0054] According to the same method as in Example 1, the product (21.2 mg, yield 45%) was obtained using 5-bromo-2-pyrrolidinylbenzaldehyde (50.8 mg, 0.2 mmol) as the reaction raw material.
[0055] 1 H NMR (400MHz, CDCl3) δ7.64(s, 1H), 7.17(d, J=8.5Hz, 1H), 7.07(d, J=8.5Hz, 1H), 6.09(s, 1H), 4.02(t ,J=6.9Hz,2H),3.00(t,J=7.4Hz,2H),2.71–2.52(m,2H).
[0056] 13 C NMR (100MHz, CDCl 3 )δ145.99, 134.87, 131.33, 122.84, 122.71, 112.38, 110.71, 92.14, 43.81, 27.79, 24.39
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com