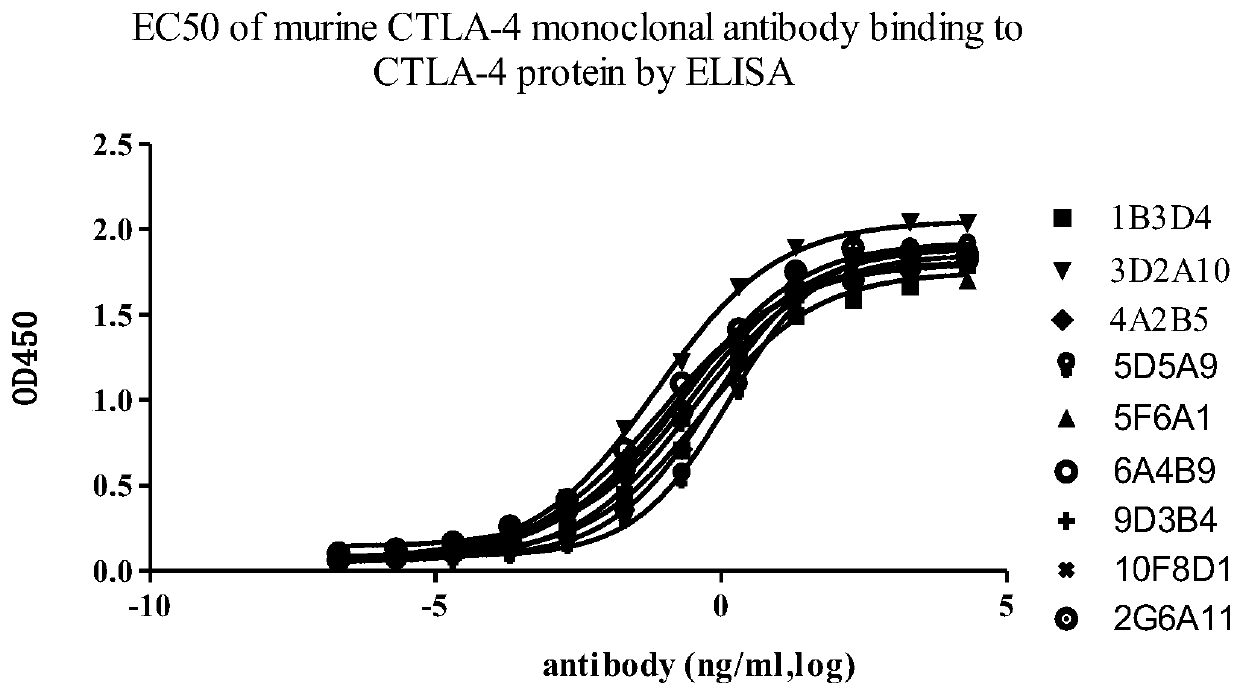
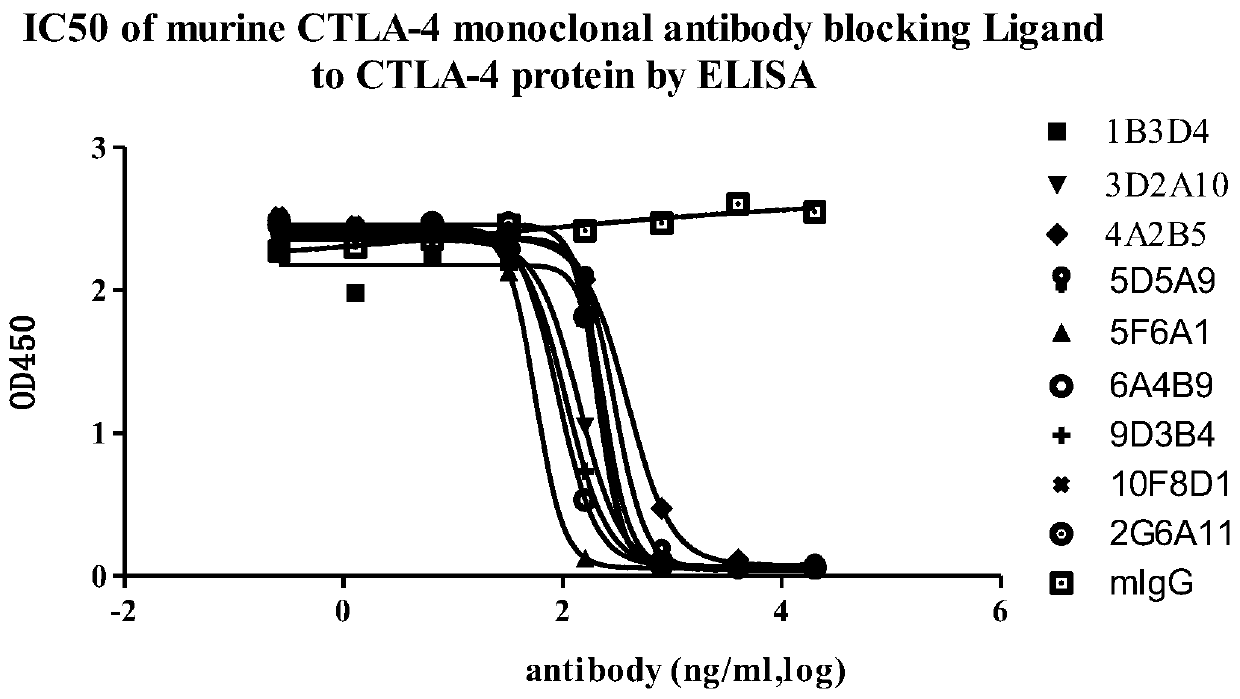
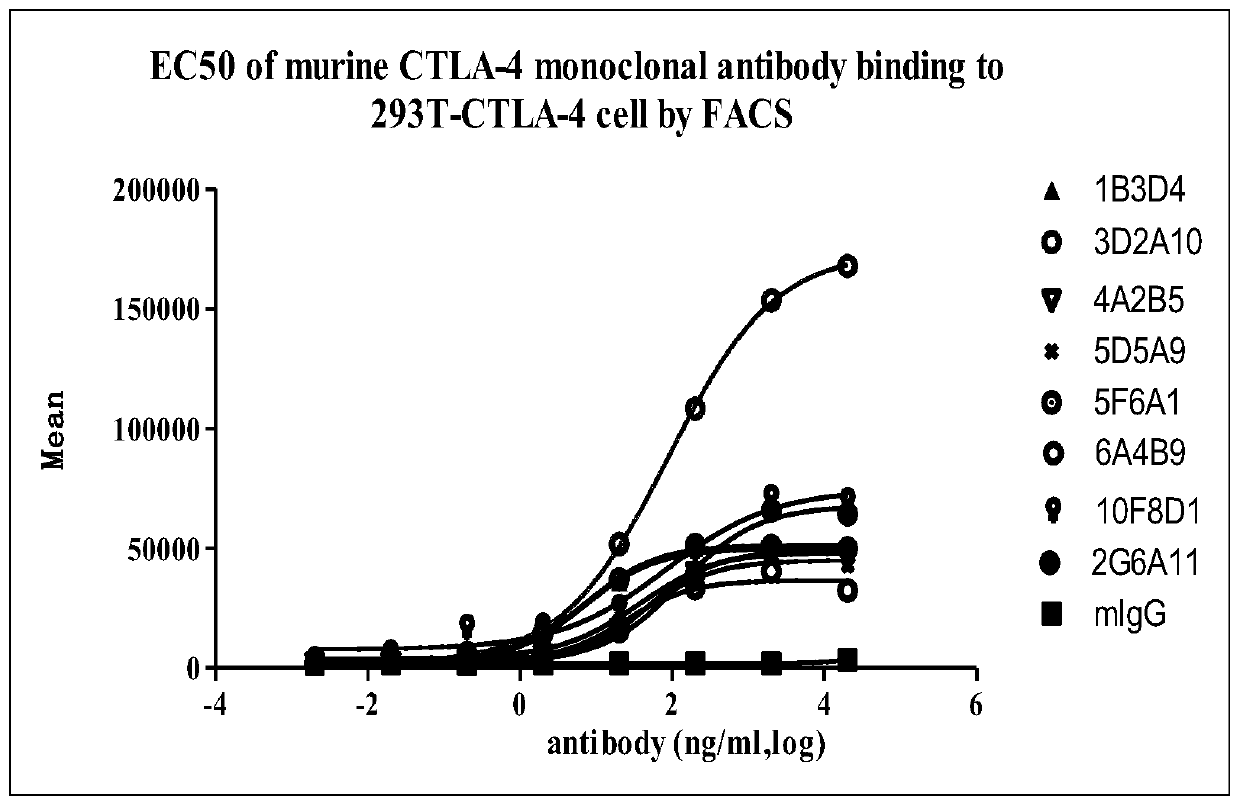
A group of anti-CTLA-4 monoclonal antibodies and medicinal application thereof
A CTLA-4, monoclonal antibody technology, applied in the field of tumor therapy and molecular immunology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The construction of the mouse antibody library includes the following steps
[0034] (1) Human CTLA-4-ECD-mFc fusion protein was used as antigen, fully emulsified with an equal volume of complete Freund's adjuvant (Sigma, Cat No: F5581), and subcutaneously immunized 6-8 week-old Balb / c mice Rats (purchased from Zhaoyan (Suzhou) New Drug Research Center Co., Ltd.), the antigen immunization dose was 20 μg / rat. Subsequently, mice were subcutaneously immunized three times with the same dose of antigen fully emulsified with incomplete Freund's adjuvant (Sigma, Cat No: F5506) every 2 weeks. After the three immunizations, the serum titer of the mice was determined, and a booster immunization was performed intraperitoneally 3 days before the fusion. Using PEG Hybri-Max (Sigma, Cat No: 7181) as a fusion agent, mouse spleen cells and SP2 / 0 cells were mixed at a ratio of 4:1. The fused cells were added to a 96-well plate (1x105 cells / well), each well containing 0.1 mL of 1X HAT ...
Embodiment 2
[0056] The experiment of the influence of CTLA-4 hybridoma antibody on peripheral blood mononuclear cells (PBMC) secreting cytokines comprises the following steps:
[0057] (1) Add lymphocyte separation solution Histopaque (Sigma, Cat No: 1077-1) into a 50 mL sterile centrifuge tube, then add an equal volume of blood, and centrifuge at 1500 rpm for 30 min at room temperature. The sample is divided into four layers in the centrifuge tube, which are plasma layer, white blood cell layer, lymphocyte separation fluid and red blood cell layer from top to bottom. Collect the white blood cell layer in the middle into a new centrifuge tube, add 5 times the volume of washing buffer (PBS+3%FBS) to mix and wash, centrifuge at 1500rpm for 10min, repeat the washing for 3 times, and resuspend the cells in washing buffer And count.
[0058] (2) After cell counting, resuspend PBMC (1×10 6 cells / mL). In a U-bottom-96-well plate, 100 μL of PBMC and 100 μl of different concentrations of CTLA-4...
Embodiment 3
[0060]The cloning of the variable region gene of the CTLA-4 antibody comprises the following steps: lysing the CTLA-4 monoclonal hybridoma cell line with TRIzon (Cwbiotech, Cat No: CW0580), and extracting the total RNA of the hybridoma cell. The RNA of hybridoma cells was reverse-transcribed into cDNA using HiFi Script cDNA Synthesis Kit (Cwbiotech, Cat No: CW2569). Using cDNA as a template, amplify the heavy chain of the antibody by PCR method (Kettleborough et al. (1993) Eur J Immunology 23:206-211; Strebe et al. (2010) Antibody Engineering 1:3-14) with degenerate primers and light chain variable region genes. After the PCR amplification product was connected to the T / A carrier, the DH5a competent cells were transformed, plated and cultured overnight at 37°C. Pick a single clone from the culture plate, expand the culture, extract the plasmid, and determine the gene sequence of the antibody. According to the gene sequence of the antibody, its complementary determinants (CDR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com