Pteridine aqueous organic redox flow battery
A flow battery, pteridine technology, applied in fuel cells, regenerative fuel cells, circuits, etc., can solve the problem of high cost of flow batteries, and achieve the effect of cheap source of elements, excellent kinetic performance, and rich source of elements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
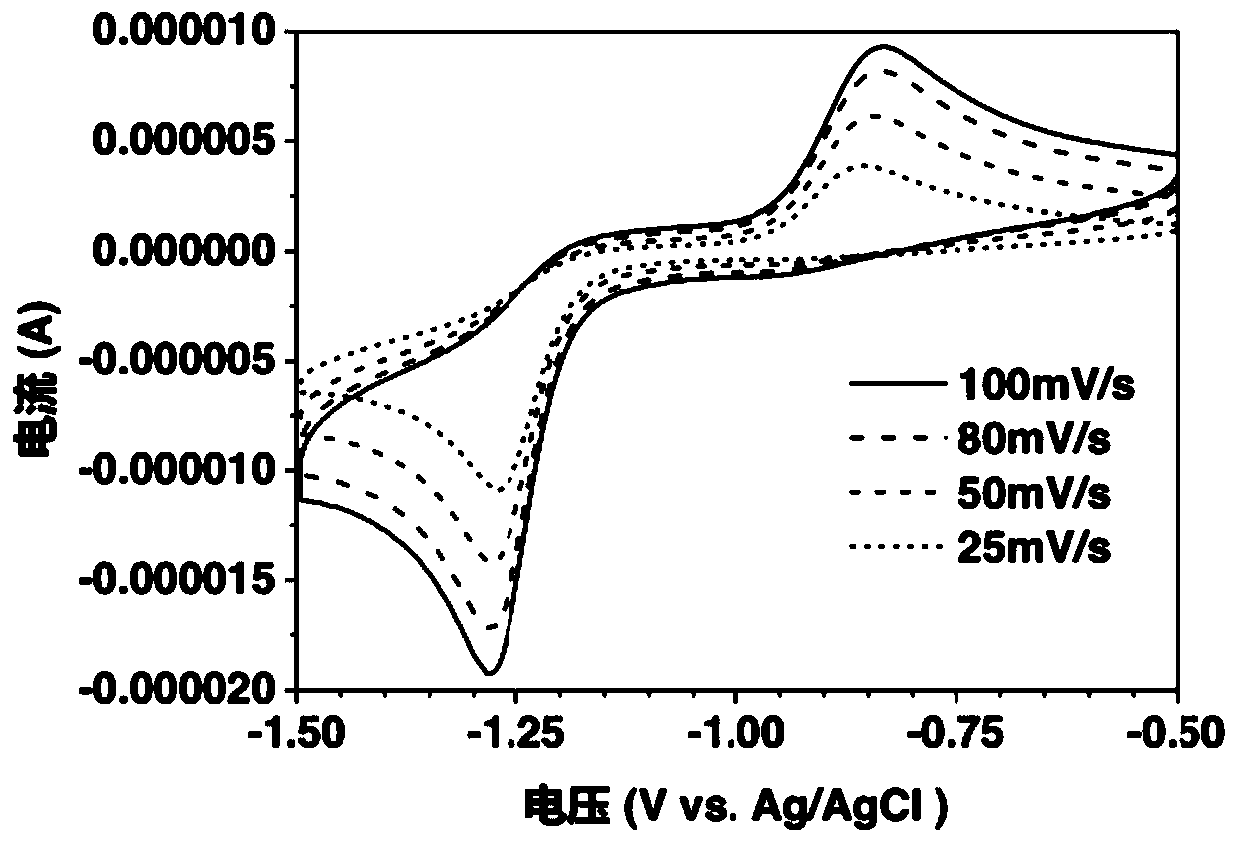
[0049] Weigh folic acid and dissolve it in 30 milliliters of 1mol / L potassium hydroxide solution, vibrate and stir, and prepare a 0.001mol / L folic acid solution after it forms a uniform solution. The electrolyte solution prepared above was subjected to a cyclic voltammetry test with a three-electrode system, wherein silver / silver chloride was used as a reference electrode, a platinum electrode was used as a counter electrode, and a glassy carbon electrode was used as a working electrode. Sweep speed is 25mV / s, 50mV / s, 80mV / s and 100mV / s.
[0050] Depend on figure 1 The middle CV data can be obtained. Under alkaline conditions, there is a pair of obvious reversible redox peaks, and its electrochemical reversibility is good. When silver / silver chloride is used as a reference electrode, the average potential of folic acid is below -1V, as The negative electrode material exhibits a relatively negative potential.
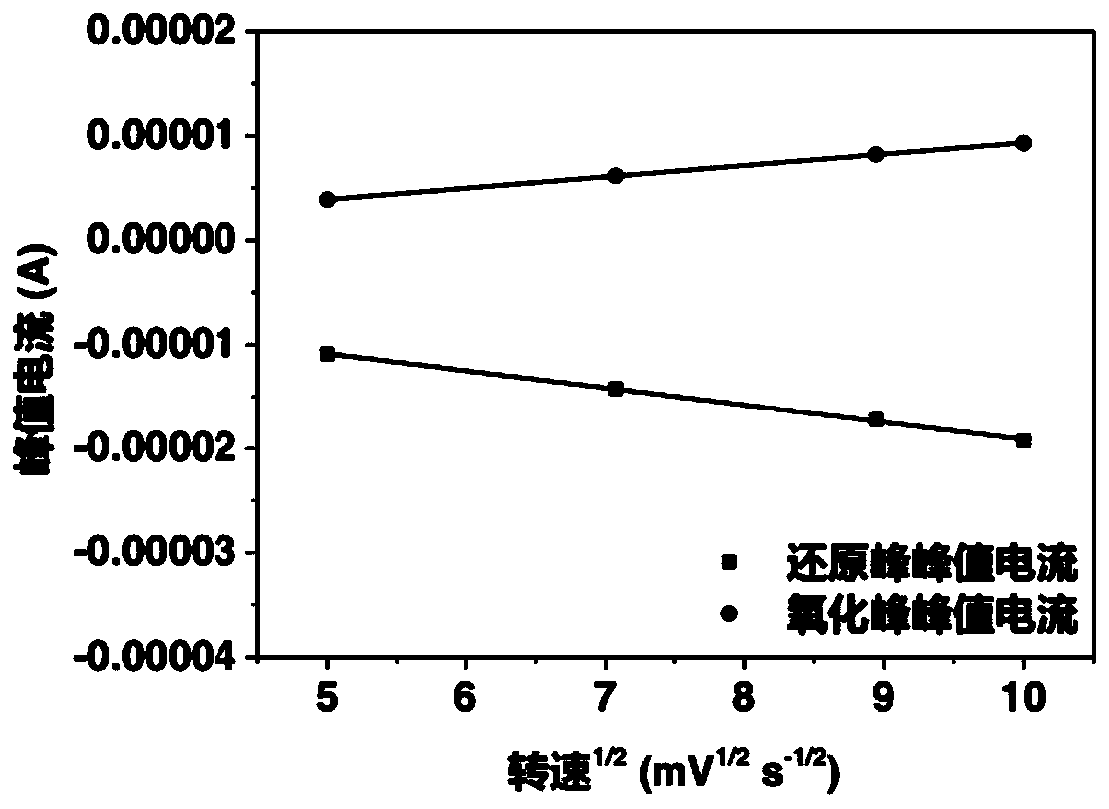
[0051] exist figure 2 In the linear fitting of the redox peak p...
Embodiment 2
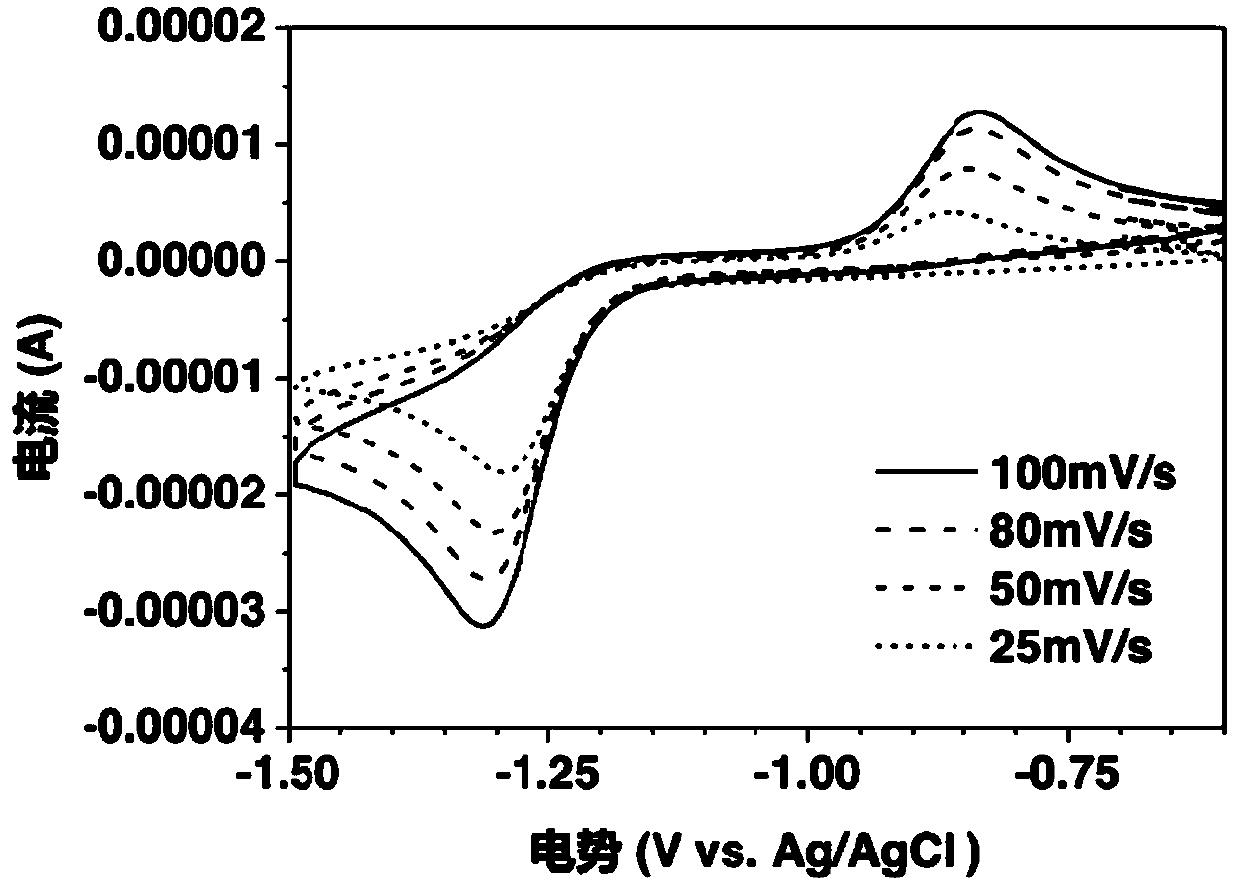
[0053] Weigh anhydrous theophylline and dissolve it in 30 milliliters of 1 mol / L potassium hydroxide solution, vibrate and stir, and prepare a 0.001 mol / L folic acid solution after it forms a uniform solution. The electrolyte solution prepared above was subjected to a cyclic voltammetry test with a three-electrode system, wherein silver / silver chloride was used as a reference electrode, a platinum electrode was used as a counter electrode, and a glassy carbon electrode was used as a working electrode. Sweep speed is 25mV / s, 50mV / s, 80mV / s and 100mV / s.
[0054] Depend on image 3 The middle CV data can be obtained. Under alkaline conditions, there is a pair of obvious reversible redox peaks, and its electrochemical reversibility is good. When silver / silver chloride is used as a reference electrode, the average potential of folic acid is below -1V, as The negative electrode material exhibits a relatively negative potential.
[0055] exist Figure 4 In the linear fitting of th...
Embodiment 3
[0057] Weigh pteroic acid and dissolve it in 30 ml of 1 mol / L potassium hydroxide solution, oscillate and stir, and prepare a 0.001 mol / L folic acid solution after it forms a uniform solution. The electrolyte solution prepared above was subjected to a cyclic voltammetry test with a three-electrode system, wherein silver / silver chloride was used as a reference electrode, a platinum electrode was used as a counter electrode, and a glassy carbon electrode was used as a working electrode. Sweep speed is 25mV / s, 50mV / s, 80mV / s and 100mV / s.
[0058] Depend on Figure 5 The middle CV data can be obtained. Under alkaline conditions, there is a pair of obvious reversible redox peaks, and its electrochemical reversibility is good. When silver / silver chloride is used as a reference electrode, the average potential of folic acid is below -1V, as The negative electrode material exhibits a relatively negative potential.
[0059] exist Image 6 In the linear fitting of the redox peak pote...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com