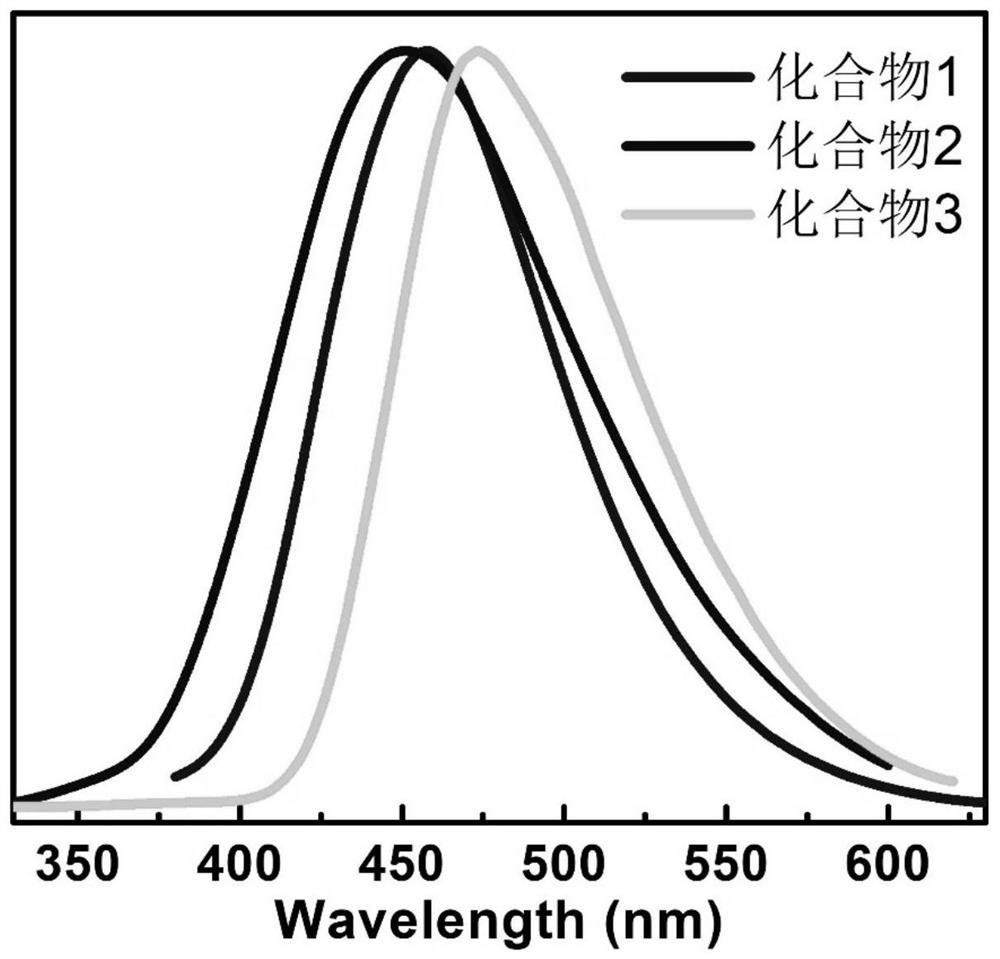
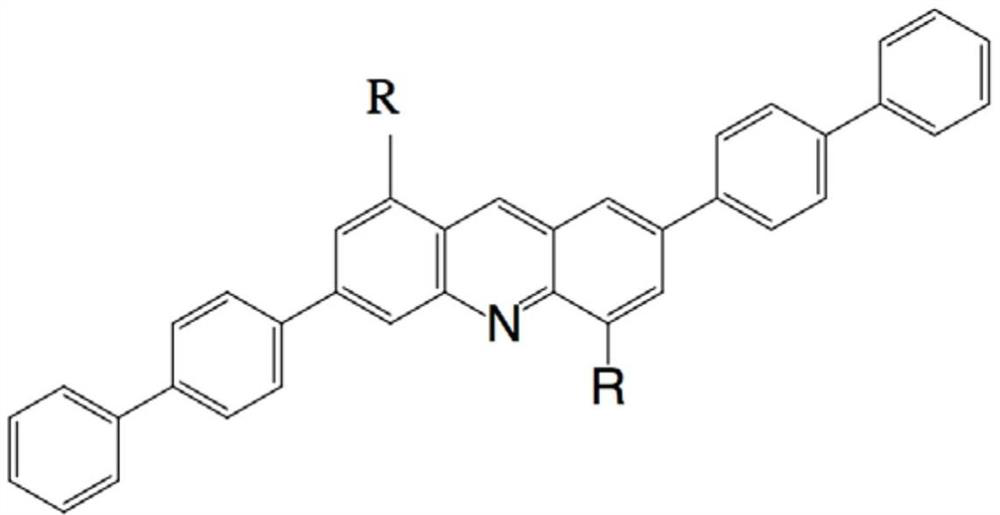
Thermally activated delayed fluorescent blue light material, its synthesis method and application
A technology of thermally activated delayed and blue-light materials, which is applied in the field of organic light-emitting materials, can solve the problems of limited applications, and achieve the effects of low singlet triplet energy level difference, high photoluminescence quantum yield, and fast reverse intersystem crossing constant
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Synthesis of thermally activated delayed fluorescent blue light material
[0029] The synthetic route is shown in the reaction formula:
[0030]
[0031] Weigh 3.19g of raw material 1 (5mmol), 4.00g of 9,10-dihydro-9,9-diphenylacridine (12 mmol), 0.18g of palladium acetate (0.8mmol) and 0.68g of tri-tert-butylphosphine Tetrafluoroborate (2.4mmol), pour in the 250mL two-necked bottle, transfer the two-necked bottle containing the reaction raw materials to the glove box, then add 2.34gNaOt-Bu (24mmol) to the two-necked bottle in the glove box ), then injected 100 mL of toluene that had been dehydrated and deoxygenated under an argon atmosphere, and reacted at 120°C for 24 hours. Cool to room temperature, pour the reaction solution into 200mL ice water, extract three times with dichloromethane, combine the organic phases, spin into silica gel, and separate and purify by column chromatography (dichloromethane:n-hexane, v:v, 1:3) to obtain Light blue powder 2...
Embodiment 2
[0034] Embodiment 2: the synthesis of thermally activated delayed fluorescent blue light material
[0035] The synthetic route reaction formula is shown as:
[0036] Weigh 3.19g raw material 1 (5mmol), 2.20g phenoxazine (12mmol), 0.18g palladium acetate (0.8 mmol) and 0.68g tri-tert-butylphosphine tetrafluoroborate (2.4mmol), pour into 250mL di transfer the two-necked bottle containing the reaction materials to the glove box, then add 2.34 gNaOt-Bu (24mmol) to the two-necked bottle in the glove box, inject 100mL of NaOt-Bu (24mmol) into the two-necked bottle under an argon atmosphere, and remove the water beforehand. Oxygen toluene, reacted at 120°C for 24 hours. Cool to room temperature, pour the reaction solution into 200mL ice water, extract three times with dichloromethane, combine the organic phases, spin into silica gel, and separate and purify by column chromatography (dichloromethane:n-hexane, v:v, 1:3) to obtain Light blue powder 2.1g, yield 49%.
[0037] The obtai...
Embodiment 3
[0039] Embodiment 3: the synthesis of thermally activated delayed fluorescent blue light material
[0040] The synthetic route is shown in the reaction formula:
[0041]Weigh 3.19g raw material 1 (5mmol), 2.34g3,6-dimethylcarbazole (12mmol), 0.18g palladium acetate (0.8mmol) and 0.68g tri-tert-butylphosphine tetrafluoroborate (2.4mmol) Pour it into a 250mL two-necked bottle, transfer the two-necked bottle containing the reaction materials to the glove box, then add 2.34g NaOt-Bu (24mmol) to the two-necked bottle in the glove box, and inject 100mL of Toluene, which had been dehydrated and deoxygenated in advance, was reacted at 120°C for 24 hours. Cool to room temperature, pour the reaction solution into 200mL ice water, extract three times with dichloromethane, combine the organic phases, spin into silica gel, and separate and purify by column chromatography (dichloromethane:n-hexane, v:v, 1:3) to obtain Light blue powder 2.6g, yield 60%.
[0042] The obtained product, i.e....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com