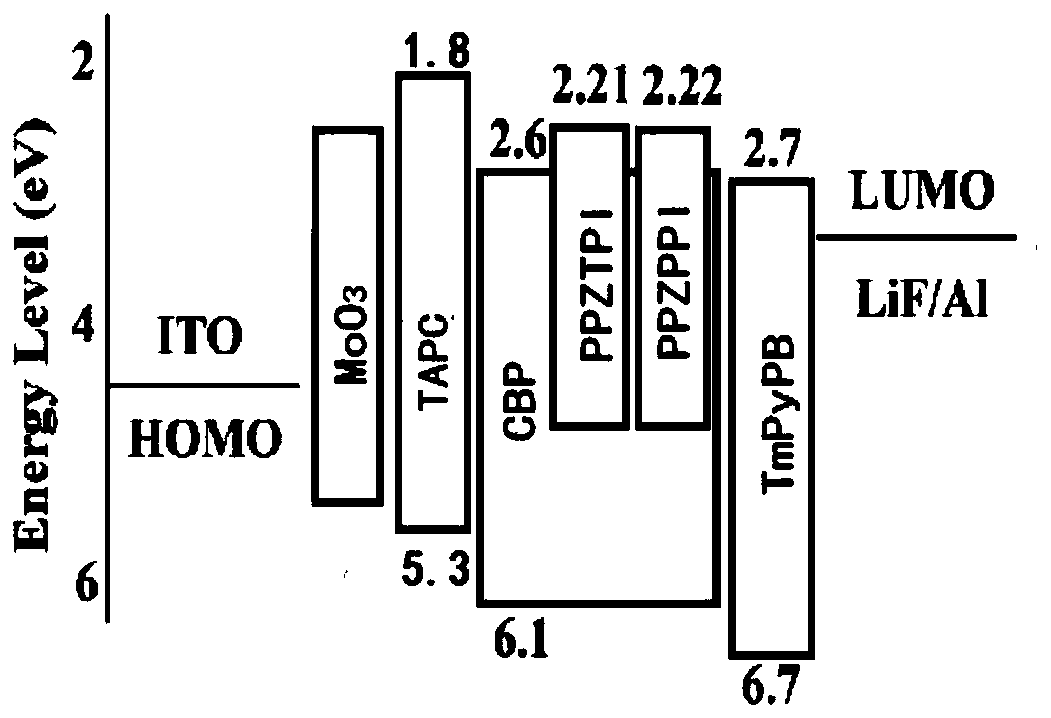
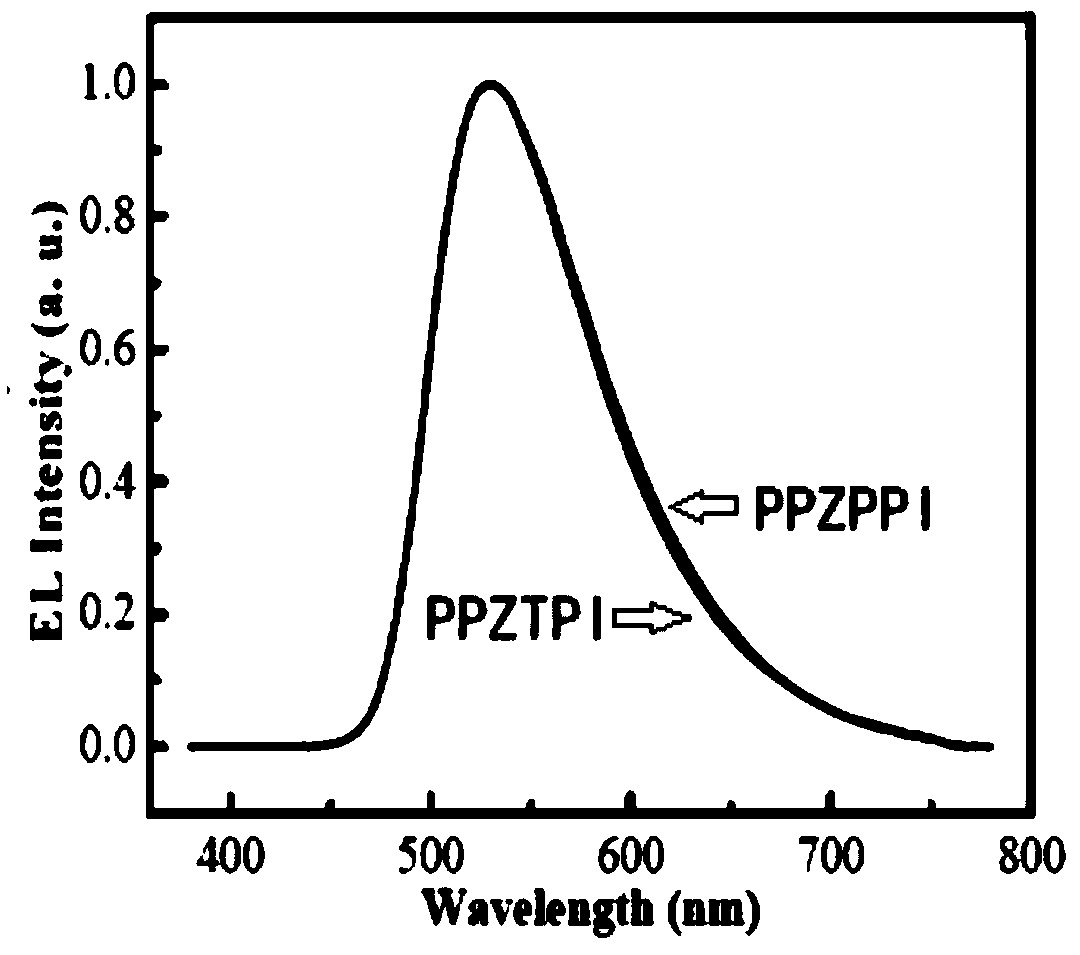
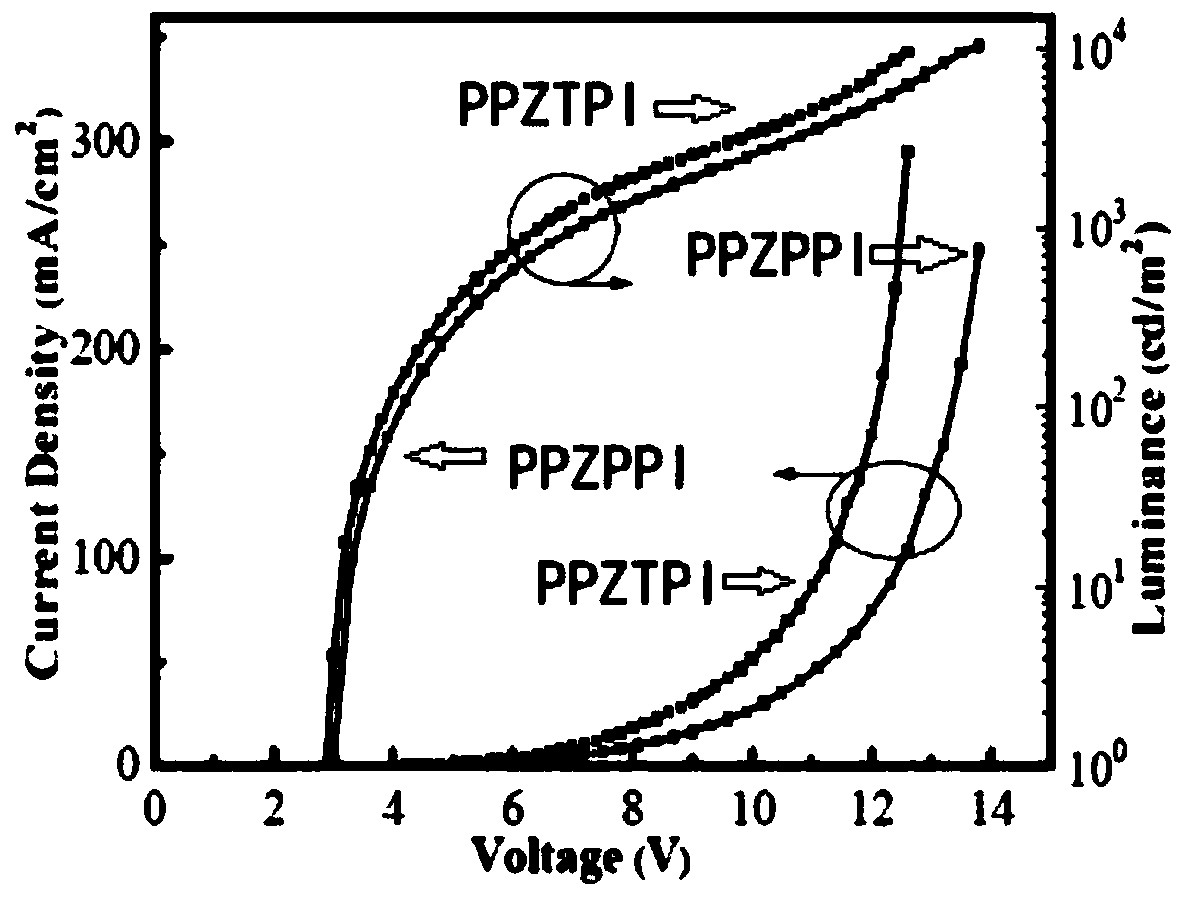
Thermally activated delayed fluorescence material with phenanthroimidazole structure, preparation method of material and application of material
A technology of thermally induced delayed fluorescence and phenanthroimidazole, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve problems such as efficiency roll-off
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: The present invention is that the above compound 1 (PPZPPI) can be synthesized by the following method.
[0086]
[0087] (1) Dissolve phenanthrenequinone (31.23g, 150mmol), aniline (16.76g, 180mmol), 4-bromobenzaldehyde (33.30g, 180mmol), ammonium acetate (23.12g, 300mmol) in a 500ml three-necked flask in 300mL Add acetic acid to the reactor, under nitrogen atmosphere, heat up to 115°C and react for 12 hours, the liquid phase monitors the completion of the reaction, cool to room temperature, wash with water twice, filter, and recrystallize twice with ethyl acetate to obtain 78.35g intermediate Body a, yield 85%;
[0088] (2) Dissolve phenazine (36.04g, 200mmol) in 200mL ethanol and add it to a 1000mL reactor, blow nitrogen into it, heat up to 85°C, dissolve sodium dithionite (174.10g, 1000mmol) in 400mL pure water The liquid funnel was slowly added dropwise to the reactor. After the dropwise addition, stirred for 30 minutes, cooled to room temperature...
Embodiment 2
[0090] Example 2: The above compound 2 (PPZTPI) of the present invention can be synthesized by the following method.
[0091]
[0092] (1) In a 500ml three-necked flask, phenanthrenequinone (31.23g, 150mmol), 4-tert-butylaniline (26.86g, 180mmol), 4-bromobenzaldehyde (33.30g, 180mmol), ammonium acetate (23.12g, 300mmol ) was dissolved in 300mL of acetic acid and added to the reactor, under a nitrogen atmosphere, the temperature was raised to 115°C for 10 hours, and the reaction was completed by liquid phase monitoring, cooled to room temperature, washed twice with water, filtered, and recrystallized twice from ethyl acetate, namely 67.48g of intermediate a can be obtained with a yield of 89%.
[0093] (2) The synthesis method of intermediate b 5,10-dihydrophenazine is the same as in Example 1.
[0094] (3) In a 500mL three-necked flask, add 5,10-dihydrophenazine (18.22g, 100mmol), bromobenzene (15.70g, 100mmol), potassium carbonate (41.46g, 300mmol), crown ether (2.64g, 10...
Embodiment 3
[0095] Example 3: The above compound 7 of the present invention can be synthesized by the following method.
[0096]
[0097] (1) In a 500ml three-necked flask, phenanthrenequinone (31.23g, 150mmol), 4-(9H-carbazol-9-yl)aniline (46.49g, 180mmol), 4-bromobenzaldehyde (33.30g, 180mmol), Ammonium acetate (23.12g, 300mmol) was dissolved in 300mL of acetic acid and added to the reactor. Under a nitrogen atmosphere, the temperature was raised to 115°C for 12 hours. The reaction was completed by liquid phase monitoring, cooled to room temperature, washed twice with water, filtered, and ethyl acetate The ester was recrystallized twice to obtain 78.35 g of intermediate a with a yield of 85%.
[0098] (2) The synthesis method of intermediate b 5,10-dihydrophenazine is the same as in Example 1.
[0099] (3) In a 500mL three-necked flask, add 5,10-dihydrophenazine (18.22g, 100mmol), bromobenzene (15.70g, 100mmol), potassium carbonate (41.46g, 300mmol), crown ether (2.64g, 10mmol ), 200...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com