Preparation method of florfenicol intermediate
A technology of florfenicol and intermediates, applied in the field of preparation of florfenicol intermediates, can solve problems such as unsatisfactory, increased process, increased process investment, etc., to reduce raw material input costs, simple process, avoid The effect of complex processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Production of special potassium fluoride: Weigh 58 grams of potassium fluoride and dissolve it in 480 ml of methanol. After heating and refluxing for 1 hour at 65°C, the methanol is recovered under reduced pressure at 30°C. After drying in a vacuum box at ℃ for 7 hours, a fluffy cotton-like special potassium fluoride product is obtained. The product effectively increases the specific surface area of potassium fluoride, thereby greatly increasing the activity of potassium fluoride (other examples Omit this manufacturing method).
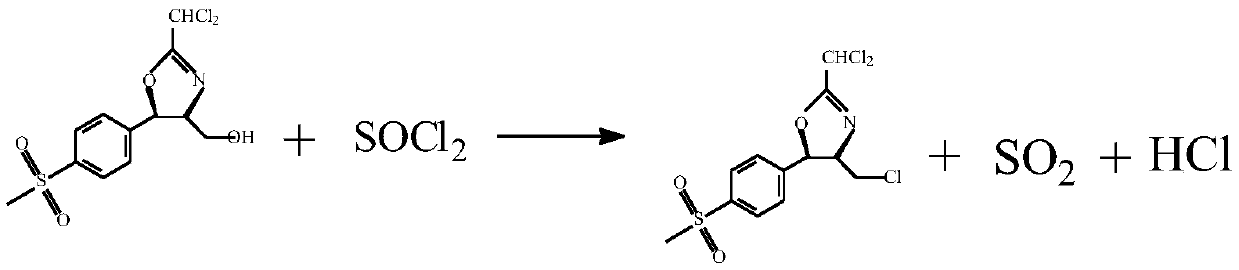
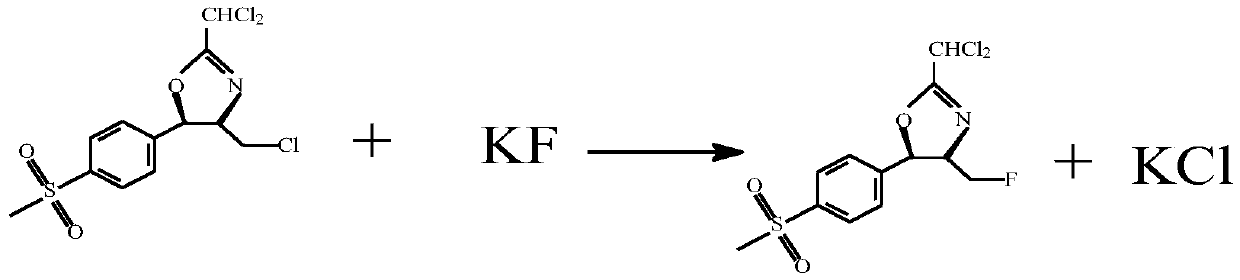
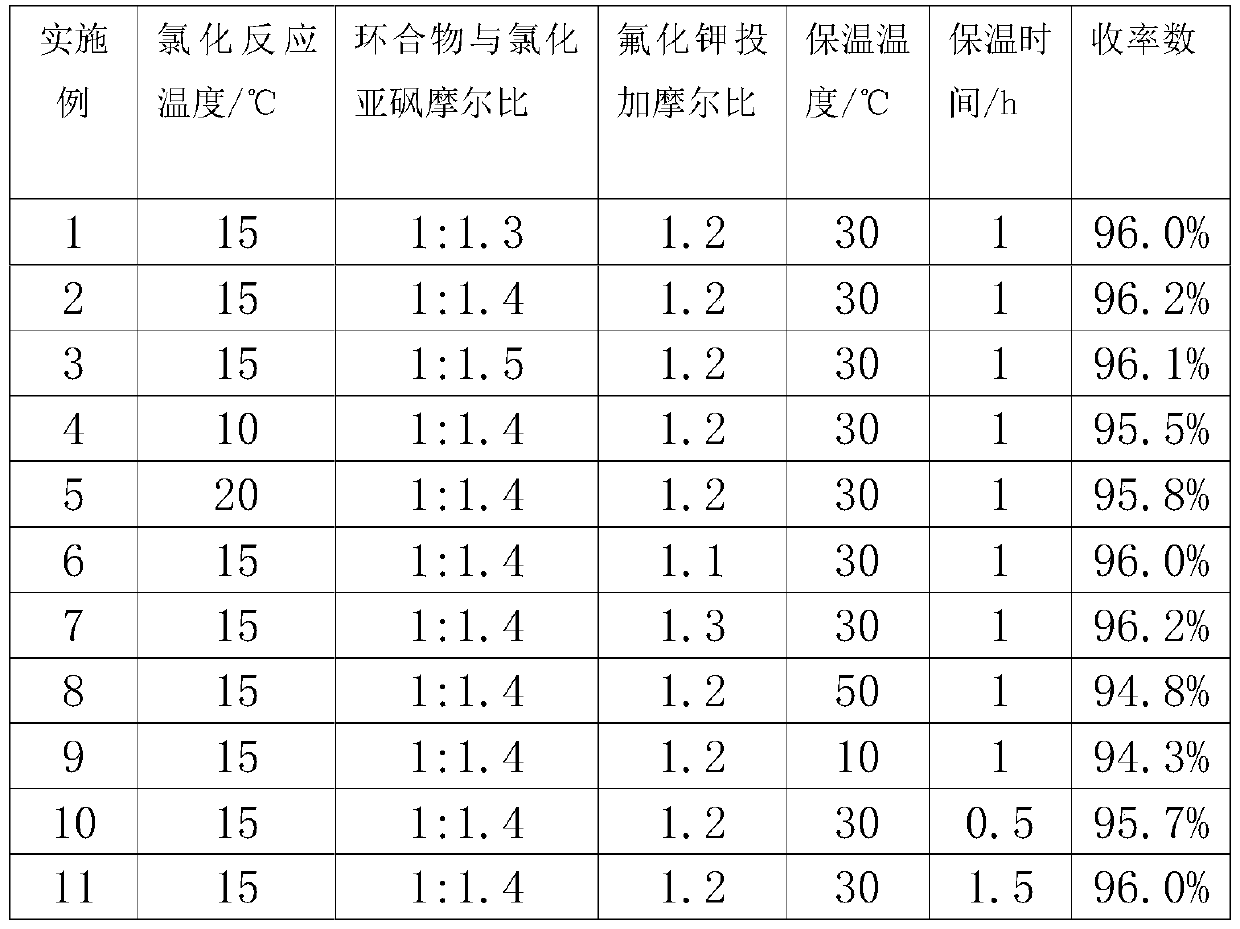
[0028] Take 10g of the prepared cyclic compound oxazoline, then take 50.25g of dichloromethane, stir to dissolve the cyclic compound in dichloromethane, and then add 4.57g of thionyl chloride dropwise to the dichloromethane of the cyclic compound In the methane solution, the temperature was kept at 15°C during the period, and the reaction was kept for 90 minutes. Then add 2.06g of special potassium fluoride to it and heat it at 30℃ for one hour...
Embodiment 2
[0030] Take 10g of the prepared cyclic compound oxazoline, then take 50.25g of dichloromethane, stir to dissolve the cyclic compound in dichloromethane, and then add 4.93g of thionyl chloride dropwise to the dichloromethane of the cyclic compound In the methane solution, the temperature was kept at 15°C during the period, and the reaction was kept for 90 minutes. Then special potassium fluoride 2.06 was added to it and the reaction was kept at 30°C for one hour. After the reaction was completed, the dichloromethane was recovered. First, the dichloromethane was recovered under normal pressure, and the temperature was raised to 65°C and then recovered under reduced pressure. The mass yield of oxazoline was 96.2%.
Embodiment 3
[0032] Take 10g of the prepared cyclic compound oxazoline, then take 50.25g of dichloromethane, stir to dissolve the cyclic compound in dichloromethane, and then add 5.28g of thionyl chloride dropwise to the dichloromethane of the cyclic compound In the methane solution, the temperature was kept at 15°C during the period, and the reaction was kept for 90 minutes. Then add 2.03g of special potassium fluoride to it and heat it at 30℃ for one hour. After the reaction is completed, the dichloromethane is recovered. First, the dichloromethane is recovered under normal pressure, and the temperature is raised to 65℃ and then recovered under reduced pressure to obtain fluoride. The mass yield of oxazoline was 96.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com