N-heterocyclic carbene-based mixed nickel (II) complex and application thereof
A nitrogen heterocycle and complex technology, applied in the field of organic synthesis preparation, can solve problems such as expensive catalysts, avoid the use of external ligands, be beneficial to large-scale synthesis and use, and have the effects of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment one: Ni[P(OEt) 3 ]{[RNC(CH 3 )C(CH 3 )NR]C}Br 2 (R = 2,4,6-trimethylphenyl) synthesis
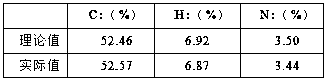
[0034] Under argon protection, nitrogen heterocyclic carbene [RNC(CH 3 )C(CH 3 )NR]C (0.3325 g, 1.0 mmol) was added to a tetrahydrofuran solution of di(triethylphosphite)nickel(II) bromide (0.5508 g, 1.0 mmol), reacted at room temperature for 3 hours, and removed the solvent in vacuo , wash the residue with n-hexane, extract the residue with toluene, transfer the supernatant and remove the solvent toluene to obtain a red solid as a divalent nickel (II) complex with a yield of 87%.
[0035] The product was subjected to elemental analysis, and the results are shown in Table 1:
[0036]
[0037] The product was characterized by NMR, and the results are as follows:
[0038]Dissolve the product in C 6 D. 6 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 ): δ 7.06 (s, 4...
Embodiment 2
[0039] Embodiment two: Ni[P(OEt) 3 ]{[RNC(CH 3 )C(CH 3 )NR]C}Br 2 (R = 2,6-Diisopropylphenyl) Synthesis
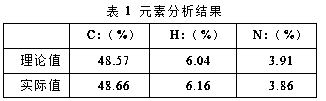
[0040] Under argon protection, nitrogen heterocyclic carbene [RNC(CH 3 )C(CH 3 )NR]C (0.4167 g, 1.0 mmol) was added to a tetrahydrofuran solution of bis(triethylphosphite) nickel(II) bromide (0.5508 g, 1.0 mmol), reacted at room temperature for 3 hours, and removed the solvent in vacuo , wash the residue with n-hexane, extract the residue with toluene, transfer the clear liquid and remove the solvent toluene to obtain a red solid as a divalent nickel (II) complex with a yield of 85%.
[0041] Carry out elemental analysis to product, the result is shown in the following table:
[0042]
[0043] The product was characterized by NMR, and the results are as follows:
[0044] Dissolve the product in C 6 D. 6 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 ): δ 7.53 (t,...
Embodiment 3
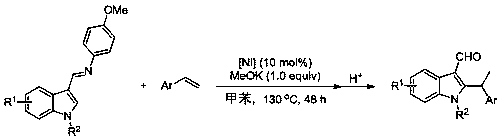
[0045] Embodiment three: Ni[P(OEt) 3 ]{[RNC(CH 3 )C(CH 3 )NR]C}Br 2 (R = 2,4,6-trimethylphenyl)-catalyzed hydroheteroarylation of N-methylindole-3-formaldehyde imine with styrene
[0046] Under the protection of argon, the catalyst (35.9 mg, 0.05 mmol, 10 mol%), potassium methylate (35.1 mg, 0.5 mmol), N-methylindole-3-formaldehyde imine (132.2 mg, 0.5 mmol), styrene (86 microliters, 0.75 mmol), toluene (1.5 ml) as solvent, at 130 o C for 48 hours, stop the reaction with water, add dilute hydrochloric acid (2 mol / L, 1 ml) for acidification, the reaction product is extracted with ethyl acetate, separated and purified by column chromatography (the volume ratio of ethyl acetate / petroleum ether is 1:5 mixed solvent as developer), the yield was 95%.
[0047] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 ): δ 10.31 (s, 1H), 8.43 (dd, J = 6.1, 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com