Nano preparation loaded with anti-pulmonary fibrosis medicament and immunomodulator and preparation method thereof
An immunomodulator and pulmonary fibrosis technology, which is applied in the field of nano-preparation and its preparation, can solve the problem of lack of effective therapeutic drugs or treatment systems for pulmonary fibrosis of nano-preparation, and achieve inhibition of fibroblast activation, reduction of collagen secretion, The effect of prolonging the cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
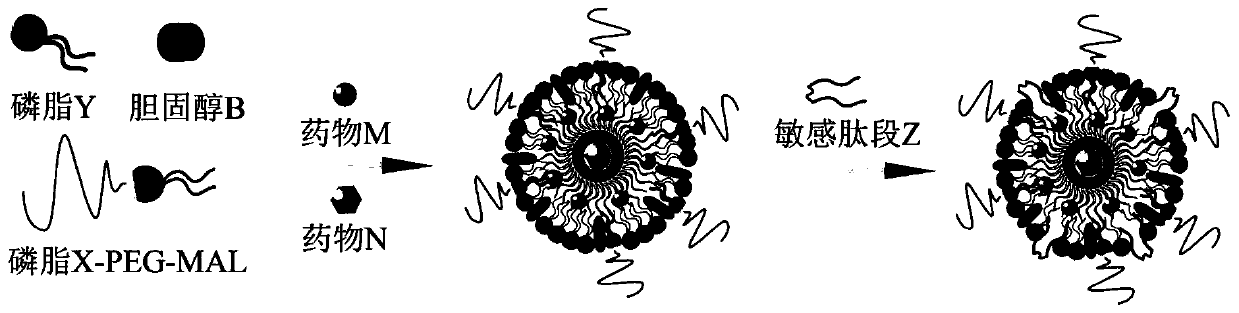
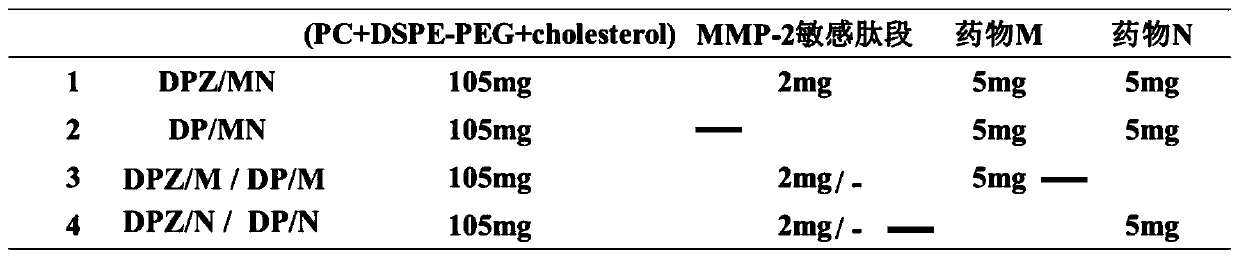
[0061] Embodiment 1 Synthesis and preparation of nano-preparation components, such as figure 1 Shown is a schematic flow chart for the preparation of DPZ / MN nano-preparations, each preparation feeding such as figure 2 Shown:
[0062] 1. Preparation of nanoparticles containing DSPE-PEG-MAL, soybean lecithin (PC), and cholesterol containing sensitive peptide DPZ loaded with double drugs
[0063] 1. Preparation of sensitive peptide DPZ-loaded double-drug nanoparticles
[0064] First, the anti-hydrophobic pulmonary fibrosis drug M, X-PEG-MAL, phospholipid Y, sensitive peptide DPZ and cholesterol are dissolved in an organic solvent and mixed. The hydrophilic drug N was dissolved in the water phase, and the nanoparticles loaded with anti-pulmonary fibrosis drugs and immunomodulators were prepared by the film dispersion method; the MMP-2 in the liposome could realize the rapid release of the drug in the fibrotic microenvironment , so that the drug can take effect quickly to achie...
Embodiment 2
[0101] Example 2 The release of nano-preparations under the condition of high expression of MMP-2
[0102] Prepare DPZ / (nintedanib / colchicine), DPZ / (nintedanib), DPZ / (colchicine), DP / (nintedanib / colchicine) according to the method described in Example 1 , DP / (nintedanib), DP / (colchicine) nano preparations. After the nano-preparation was concentrated to 500 μL, 10 μg / mL MMP-2 matrix enzyme was added, and then placed at 37 ºC for 24 h, the particle size analyzer was used to measure the drug release rate of the nano-preparation under the condition of high MMP-2 expression.
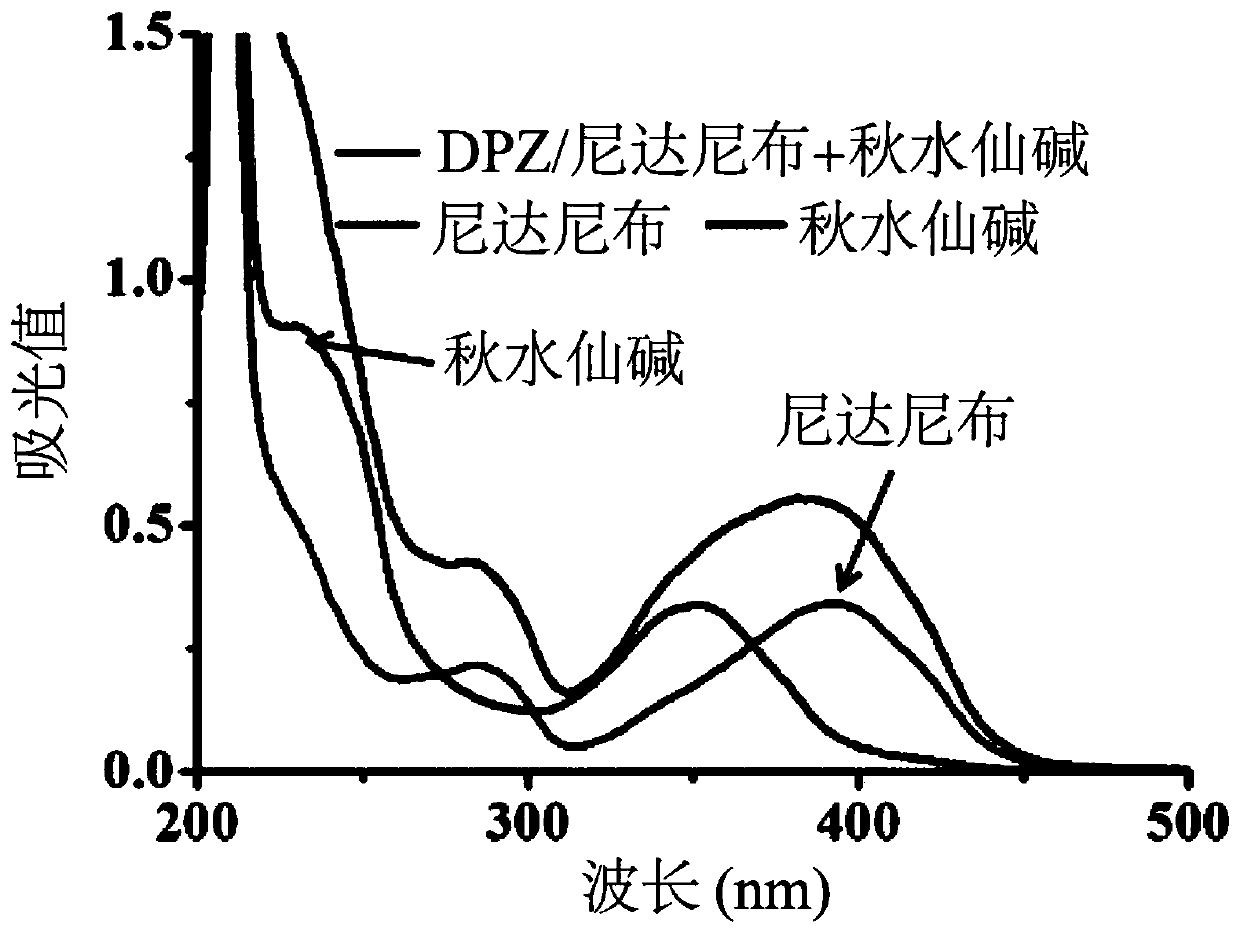
[0103] (1) The MMP-2 matrix enzyme was added to the nano-preparation containing MMP-2, the nano-preparation without MMP-2, the nano-preparation containing MMP-2, and the nano-preparation containing MMP-2 were not tested by ultraviolet spectrophotometer. The nano-preparation with MMP-2 matrix enzyme and colchicine were scanned at the full wavelength of 200nm-800nm to investigate the drug release rate under ...
Embodiment 3
[0105] Example 3 Using coumarin 6 as a model drug to investigate the escape ability of nano-preparations in primary fibroblasts
[0106] Nanoformulations of DP / coumarin 6 were prepared as described in Example 1. Primary fibroblasts were divided into 2 × 10 4 / mL inoculated in a confocal culture dish at 37 °C, 5% C0 2 After 24 h of adherent growth in the cell culture incubator, the culture medium was sucked off, and 1 mL (10 μg / mL) of coumarin 6 nanometer preparation was added, and incubated with fibroblasts for 30 min, 1 h, and 4 h, respectively. Wash three times with PBS, add cell fixative and incubate for 10 min, then wash three times with PBS. Then add lysotraker red lysosomal dye, at 37°C, 5% CO 2 After staining for 30 min under the conditions of , and washing with PBS for three times, the escape of nano-preparations in primary fibroblasts at different time points was measured using laser confocal (LSM-700).
[0107] The nano-preparation lysosome escape measured in th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com