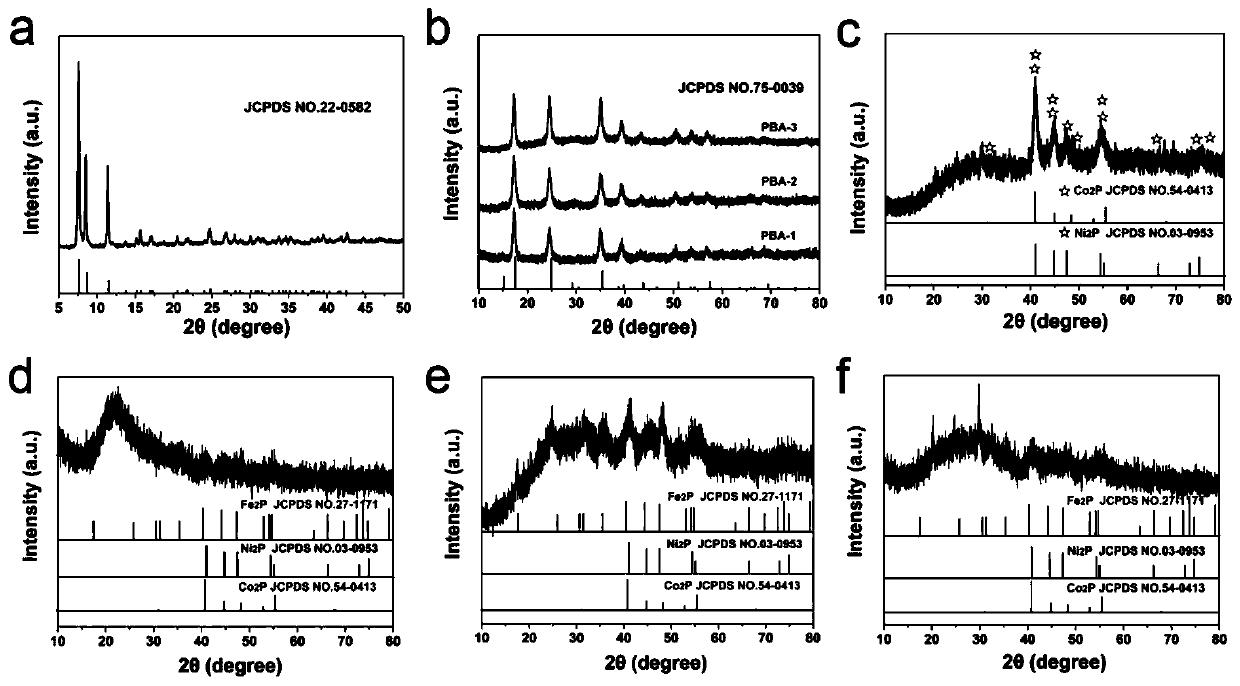
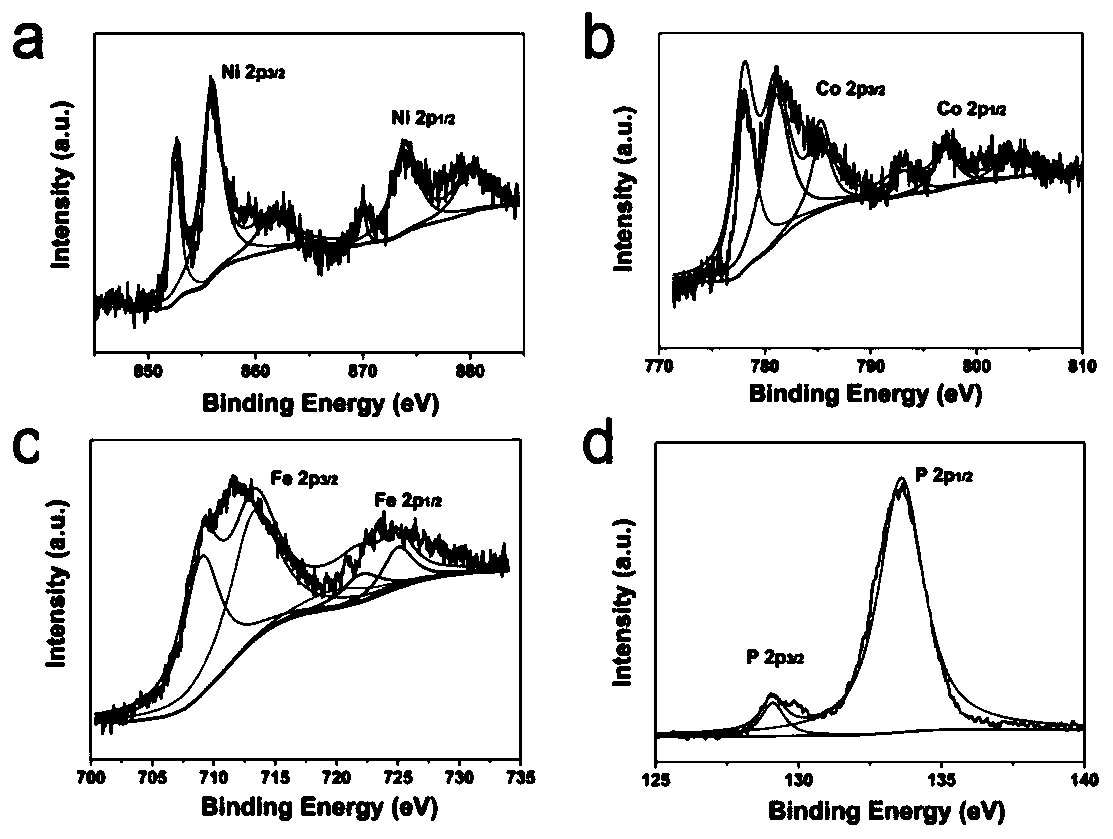
Preparation method of hollow Ni2P/Co2P/Fe2P nano-composite electrocatalyst
A technology of nanocomposite and nanocomposite materials, which is applied in the field of preparation of hollow Ni2P/Co2P/Fe2P nanocomposite electrocatalysts, which can solve the problems of general electrocatalytic oxygen evolution performance, complex synthesis process and low output, and achieve high electrocatalytic performance As well as stability, simple synthesis process, and the effect of reducing transfer internal resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Weigh 0.86g nickel acetate (Ni(CH 3 COO) 2 4H 2 O), 0.43g cobalt acetate (Co(CH 3 COO) 2 4H 2 (0) and 3g of polyvinylpyrrolidone (PVP), dissolved in 200ml of ethanol, ultrasonically dispersed to form a uniform solution, and then stirred for 30 minutes, then the above mixed solution was transferred to a round-bottomed flask, reflux reaction by condensation, through 85 ℃ , and reacted for 4 hours. After naturally cooling to room temperature, the sample was washed with deionized water and absolute ethanol, and the precipitate was dried to obtain nickel precursor nano-squares.
[0024] (2) Dissolve the nickel-cobalt precursor of 40mg in the ethanol of 5ml and disperse the ultrasound, then take 30mg of potassium ferricyanide (K 3 [Fe(CN) 6 ]) dissolved in 20ml ethanol and 20ml water, after ultrasonic dispersion will contain K 3 [Fe(CN) 6 ] The solution is poured into the nickel-cobalt precursor. React at room temperature for 10 minutes, wash the sample with deio...
Embodiment 2
[0031] (1) Weigh 0.86g nickel acetate (Ni(CH 3 COO) 2 4H 2 O), 0.43g cobalt acetate (Co(CH 3 COO) 2 4H 2 (0) and 3g of polyvinylpyrrolidone (PVP), dissolved in 200ml of ethanol, ultrasonically dispersed to form a uniform solution, and then stirred for 30 minutes, then the above mixed solution was transferred to a round-bottomed flask, reflux reaction by condensation, through 85 ℃ , and reacted for 4 hours. After naturally cooling to room temperature, the sample was washed with deionized water and absolute ethanol, and the precipitate was dried to obtain nickel precursor nano-squares.
[0032] (2) Dissolve the nickel-cobalt precursor of 40mg in the ethanol of 5ml and disperse the ultrasound, then take 40mg of potassium ferricyanide (K 3 [Fe(CN) 6 ]) dissolved in 20ml ethanol and 20ml water, after ultrasonic dispersion will contain K 3 [Fe(CN) 6 ] The solution is poured into the nickel-cobalt precursor. React at room temperature for 10 minutes, wash the sample with deio...
Embodiment 3
[0040] (1) Weigh 0.86g nickel acetate (Ni(CH 3 COO) 2 4H 2 O), 0.43g cobalt acetate (Co(CH 3 COO) 2 4H 2 (0) and 3g of polyvinylpyrrolidone (PVP), dissolved in 200ml of ethanol, ultrasonically dispersed to form a uniform solution, and then stirred for 30 minutes, then the above mixed solution was transferred to a round-bottomed flask, reflux reaction by condensation, through 85 ℃ , and reacted for 4 hours. After naturally cooling to room temperature, the sample was washed with deionized water and absolute ethanol, and the precipitate was dried to obtain nickel precursor nano-squares.
[0041] (2) Dissolve the nickel-cobalt precursor of 40mg in the ethanol of 5ml and disperse the ultrasound, then take 50mg of potassium ferricyanide (K 3 [Fe(CN) 6 ]) dissolved in 20ml ethanol and 20ml water, after ultrasonic dispersion will contain K 3 [Fe(CN) 6 ] The solution is poured into the nickel-cobalt precursor. React at room temperature for 10 minutes, wash the sample with deio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com