A marker combination and its application in the preparation of colorectal cancer diagnostic reagents
A technology of diagnostic reagents and markers, applied in the field of biomedicine, can solve problems such as no significant improvement, and achieve high accuracy, improved survival rate, high sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1 sample source and analysis
[0091] A total of 87 patients (stages I, II, III, and IV) diagnosed with colorectal cancer in the Sixth Affiliated Hospital of Sun Yat-sen University from September 2018 to December 2018 were collected, all of whom were confirmed by imaging and pathological examination. All pathological results were obtained by tissue biopsy. The patient had no serious organ disease, and had not received chemotherapy, radiotherapy or surgery. A total of 90 cases were collected as controls from a healthy population at the same time. The inclusion criteria for healthy controls were that there were no obvious abnormalities in the results of blood routine and biochemical tests, and no infectious diseases such as hepatitis B. The basic information is shown in Table 2. All blood samples were collected using EDTA anticoagulant tubes. Plasma separation was completed within 6 hours at room temperature, centrifuged at 3000 rpm for 2 minutes, and the pla...
Embodiment 2
[0096] Embodiment 2 Tumor marker content determination method
[0097] The content of RANTES in the present invention adopts enzyme-linked immunosorbent assay (ELISA) detection, selects commercial kit RayBiotech Human RANTES ELISA Kit, Biotek Elx800 microplate reader 450nm, Biotek Elx50 washer, pipette, small centrifuge tube, Ionized water, Sigmaplot analysis software, etc. The contents of CEA, CA125 and CA199 were detected by chemiluminescent enzyme immunoassay (CLEIA) in Abbott I2000.
[0098] (1) Equilibrate the kit and samples to room temperature (18-25°C);
[0099] (2) The diluent is diluted 5 times with deionized water for later use;
[0100] (3) Plasma samples were diluted 40 times for later use;
[0101] (4) Standard product preparation: Centrifuge the small tube of the standard product, then add 400 μL of 1× diluent to the small tube of the standard product, and mix well to obtain a 50 ng / mL standard product stock solution; draw 40 μL of the standard product stock ...
Embodiment 3
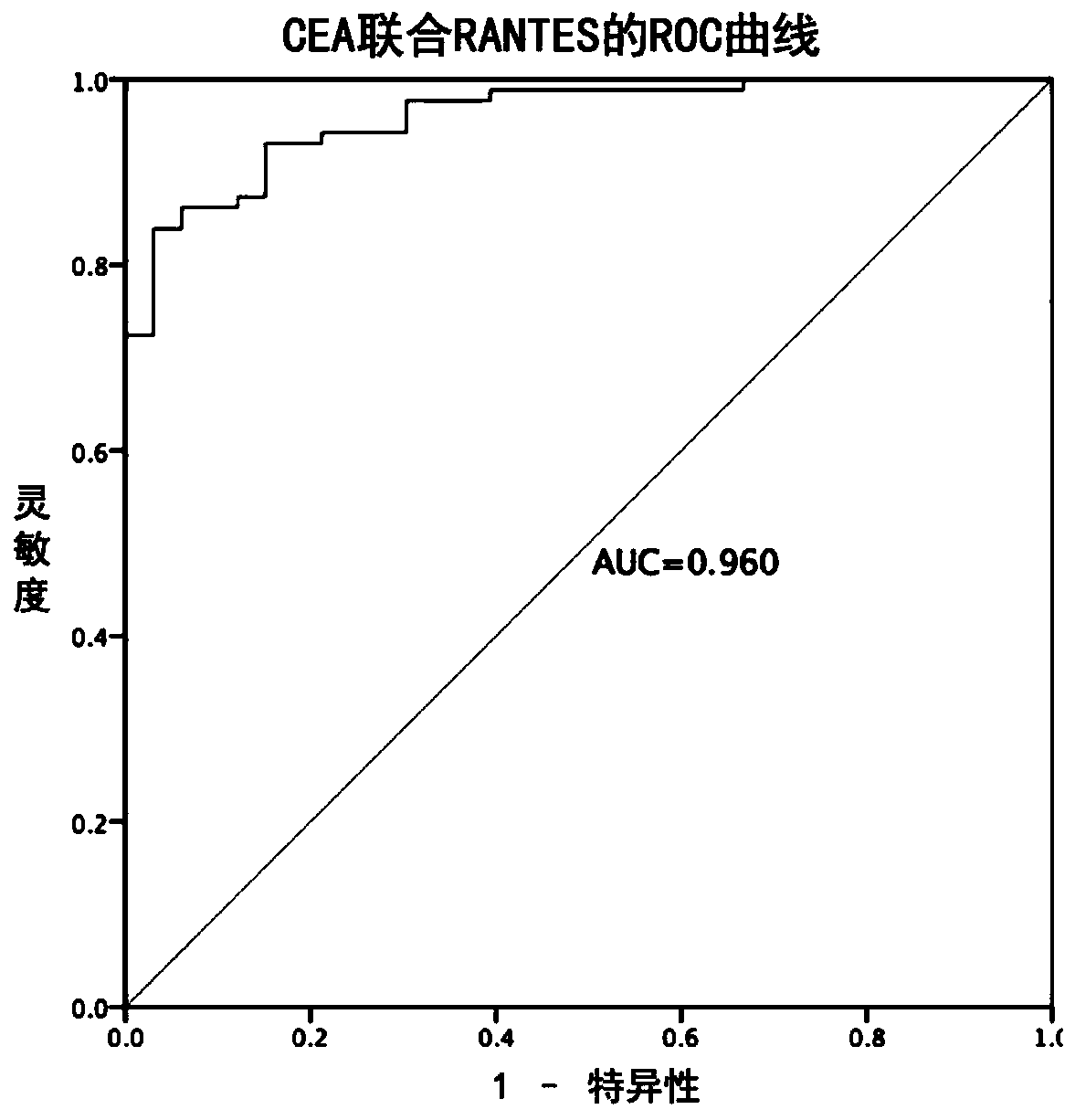
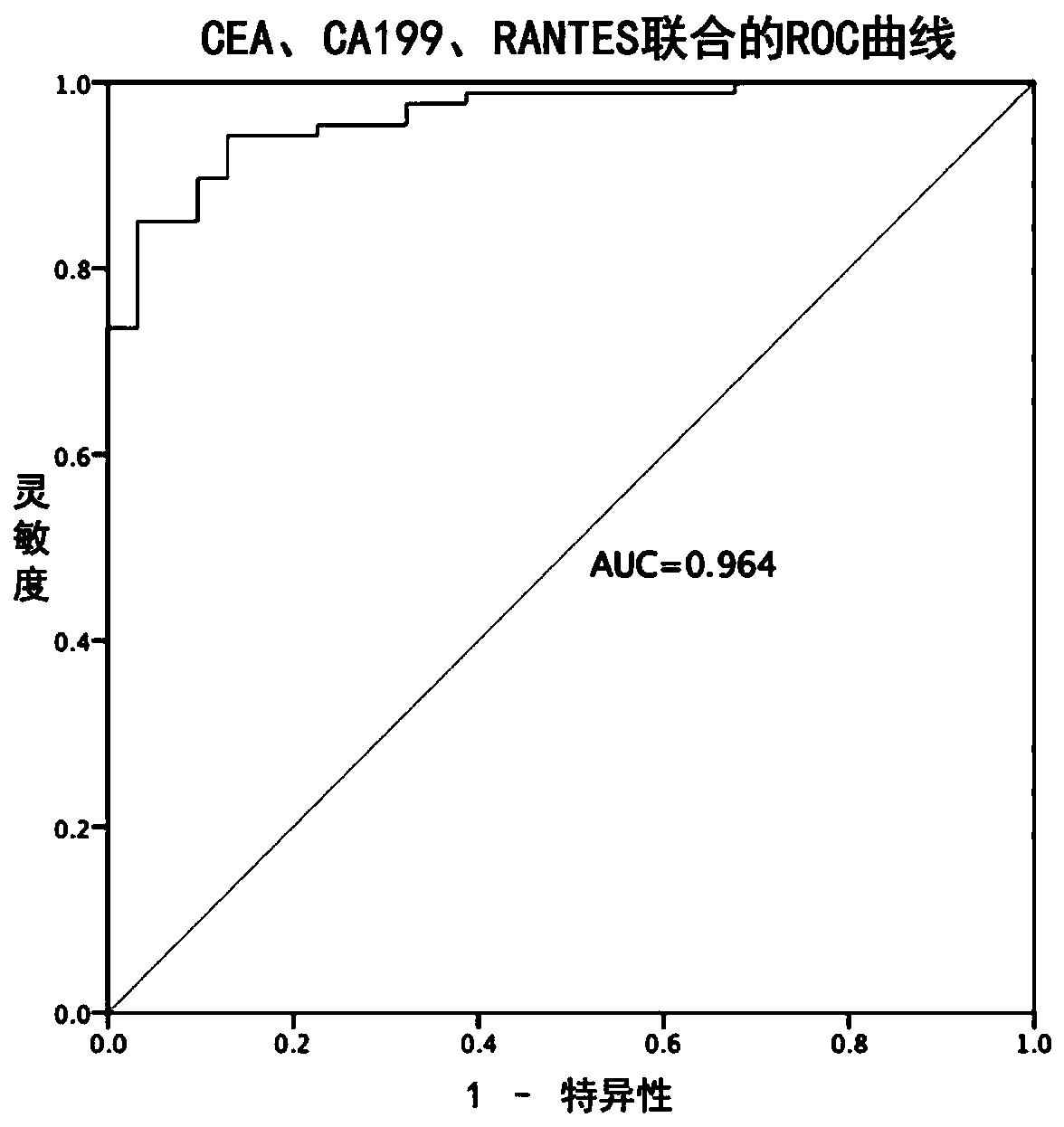
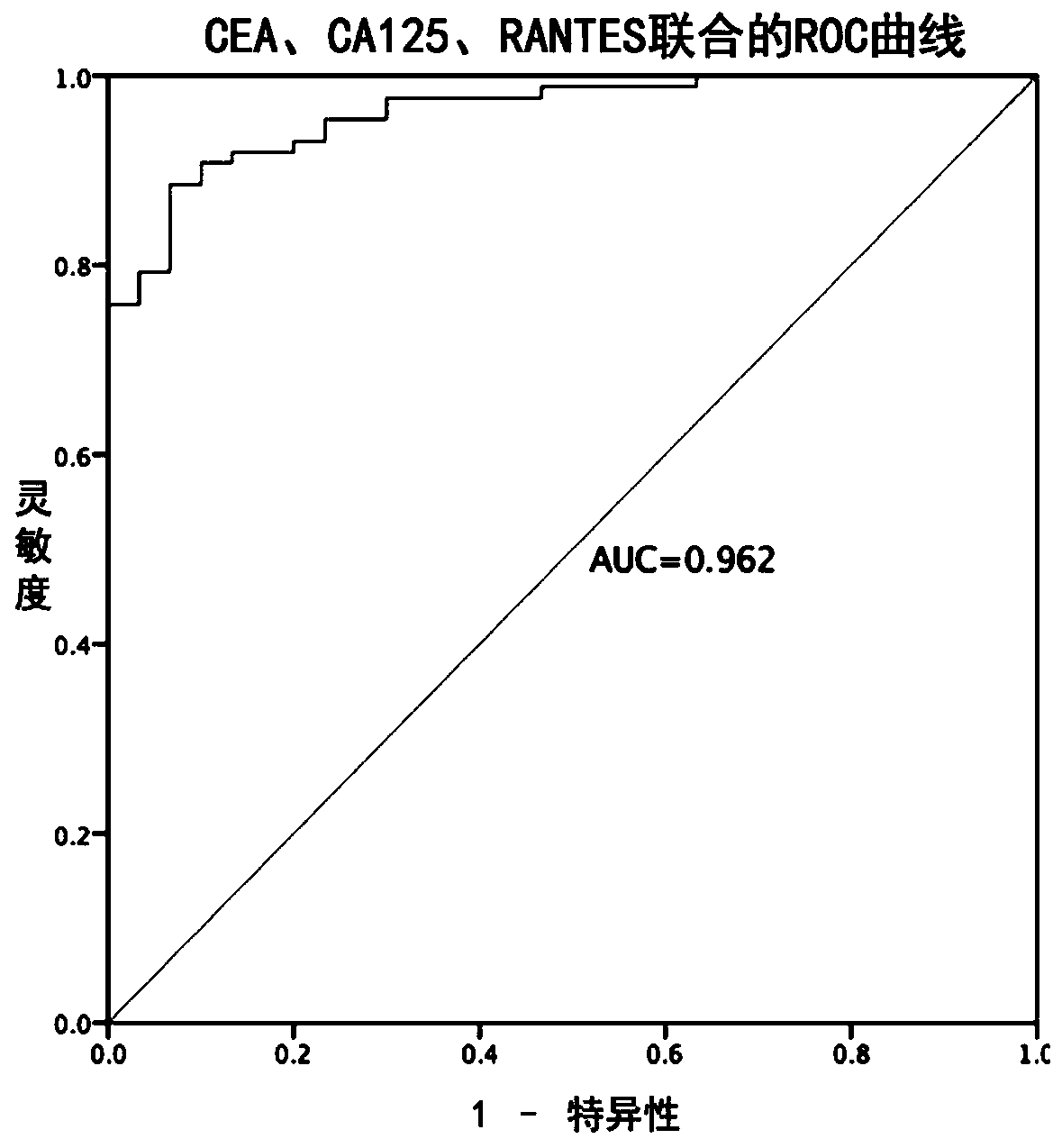
[0114] Example 3 Tumor markers to the diagnostic efficacy test results of colorectal cancer
[0115] 1. Using CEA, CA125, CA199 and RANTES as tumor markers, the corresponding Youden index and cutoff values obtained from the samples in Example 1 detected by the measurement method in Example 2 are shown in Table 3.
[0116] Table 3 Youden index maximum value and cutoff value corresponding to CEA, CA125, CA199, RANTES
[0117]
[0118] 2. Combined indicators to predict the diagnostic efficacy of CRC
[0119] CEA, CA125, CA199, and RANTES combined schemes to compare the diagnostic efficiency of colorectal cancer in the samples in Example 1. Wherein, the data are calculated for 87 colorectal cancer patients and 90 healthy controls in Example 1.
[0120] The ROC curve is obtained by Logistic regression, in which the independent variable is the corresponding index, and the dependent variable is the cancer status. The probability of each individual suffering from cancer can be ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com