Tissue engineering bionic liver lobe structure based on 3D printing of living cells and preparation method thereof
A 3D printing and tissue engineering technology, applied in the field of biomedical engineering, can solve problems such as difficulty in achieving long-term survival of large-volume artificial tissues, difficulty in forming cell function integration, and support barriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 (Temperature-controlled dual-stage etching)
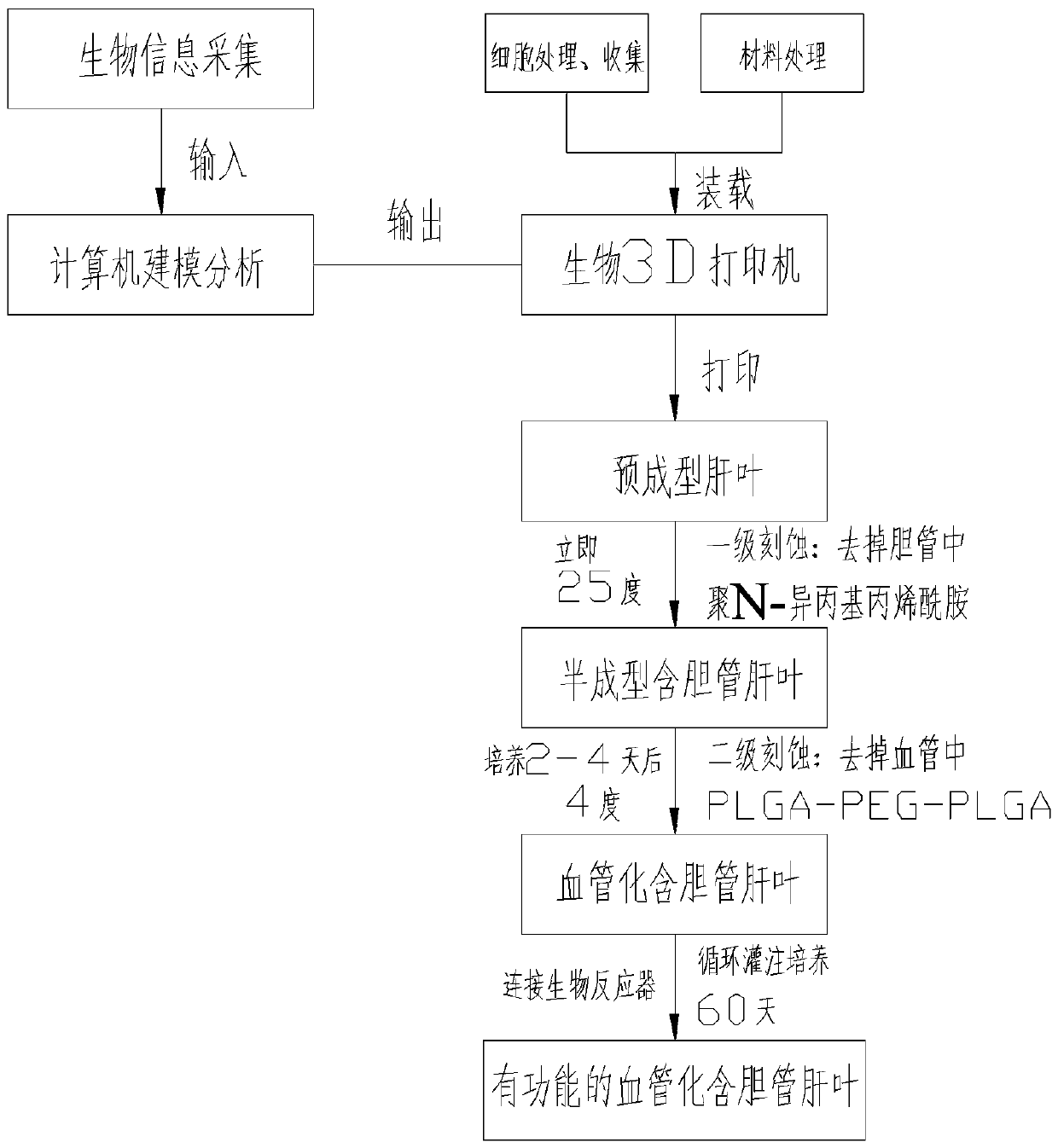
[0039] see figure 1 , this embodiment discloses a method for preparing a bionic liver lobe for tissue engineering based on living cell 3D printing, comprising the following steps:
[0040] 1. Biological information collection and modeling:
[0041] 1) Personalized collection of three-dimensional data of the internal and external structures of the liver lobe containing bile ducts and the blood circulation network of normal people through CT, nuclear magnetic resonance and micro three-dimensional scanning technology;
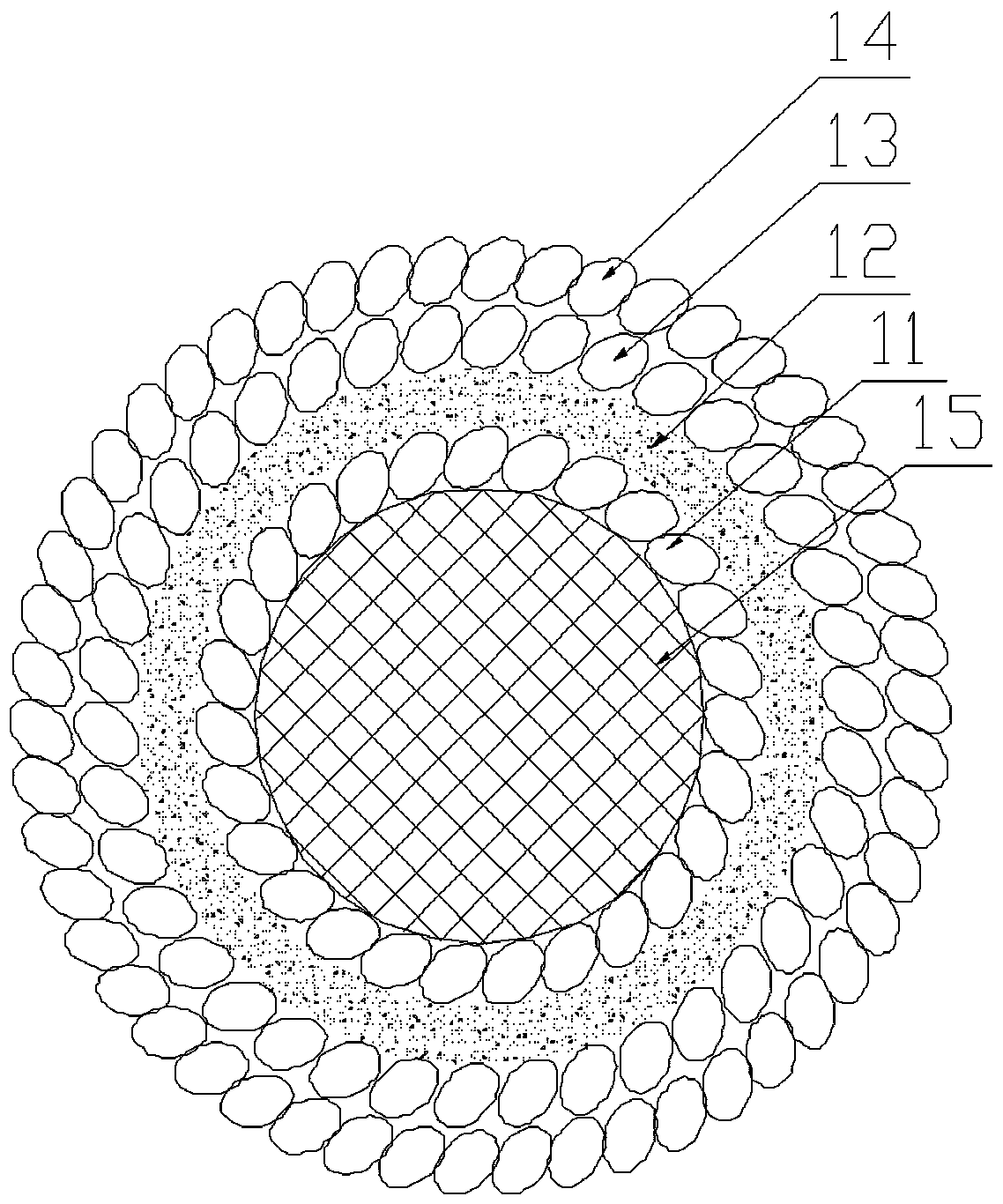
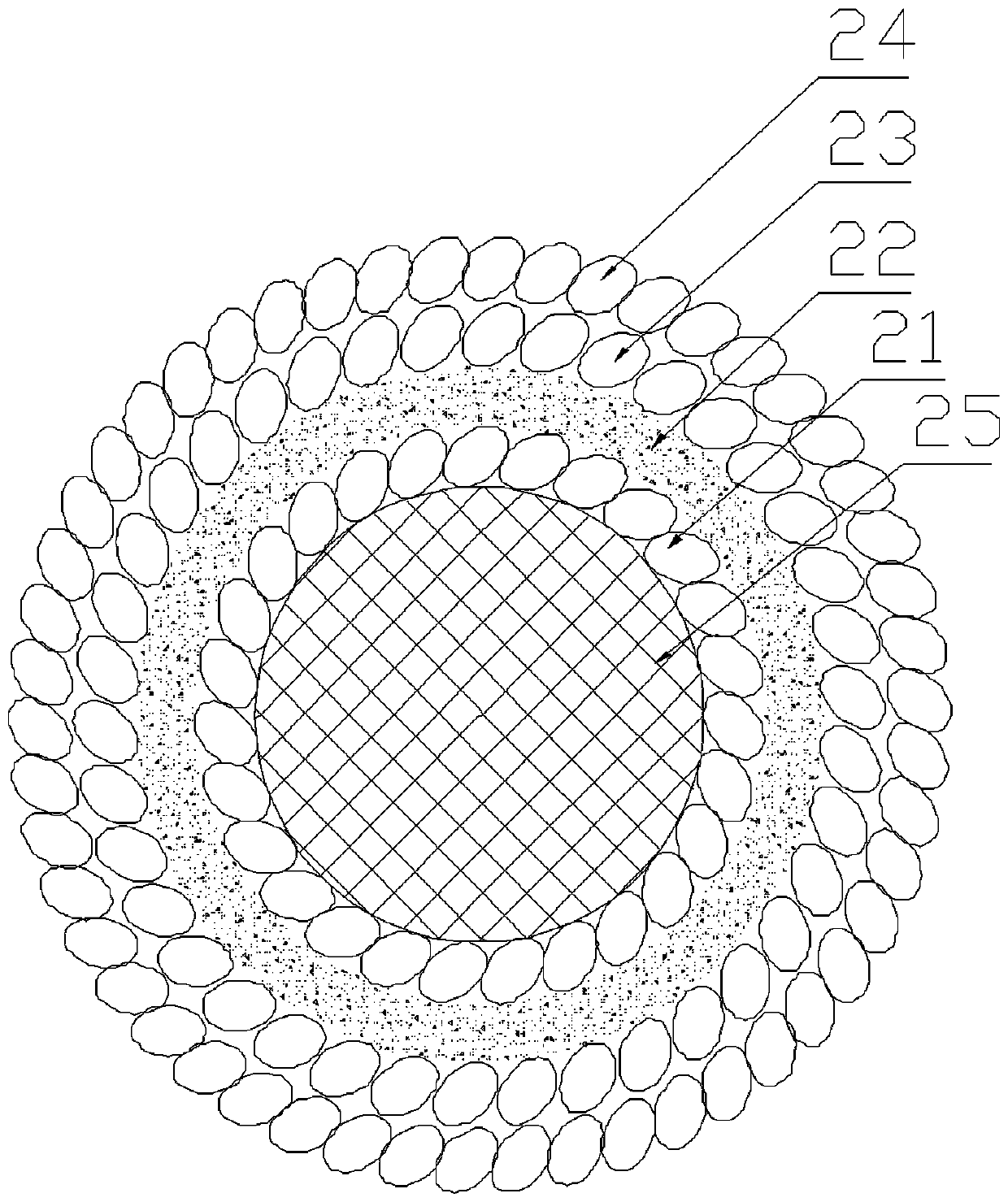
[0042] 2) Input the collected biological information into the computer software, and imitate the actual tissue appearance and microenvironment to express it as a multi-material, multi-scale geometric model. The two ends of the vascular network of the liver lobe can be designed as two main blood vessels entering and exiting for communication with The culture fluid delivery tubes are connected to form ...
Embodiment 2
[0062] Example 2 (temperature control, enzyme dual-stage etching)
[0063] The first casting agent in this embodiment is fibrinogen and thrombin. Prepare fibrinogen, thrombin and fibrinase solutions respectively. The fibrinogen solution was prepared at a concentration of 100 mg / ml, and the thrombin and fibrinase solutions at a concentration of 1 U / ml, which were stored in a 4°C refrigerator before printing. The nozzle that sprays out cell-free fibrinogen and thrombin is maintained at 4°C without a UV light source. The nozzle for ejecting fibrinogen and thrombin is divided into two layers to separate the fibrinogen and thrombin, and the fibrinogen and thrombin are mixed together after ejection.
[0064] The second molding agent in this embodiment is poly-N-isopropylacrylamide. The prepared poly-N-isopropylacrylamide solution had a concentration of 100 mg / ml and stored it in a 4 °C refrigerator before printing to keep it in a liquid state. The nozzle spraying cell-free poly-...
Embodiment 3
[0068] Example 3 (Temperature control, chelation reaction dual-stage etching)
[0069] The first casting agent of this embodiment is sodium alginate and CaCl without cells 2 . Preparation of sodium alginate, CaCl 2 , Ethylenediaminetetraacetic acid (EDTA) solution. The prepared sodium alginate mass fraction is 10%, CaCl 2 The mass fraction is 0.5%. EDTA was dissolved in NaOH at a concentration of 100 mg / ml. Spray cell-free sodium alginate and CaCl 2 The spray head is kept at 4°C without UV light source. Which sprays sodium alginate and CaCl 2 The nozzle is divided into two layers, sodium alginate and CaCl 2 Separated, sodium alginate and CaCl after spraying 2 just mixed together.
[0070] The second molding agent in this embodiment is poly-N-isopropylacrylamide. The prepared poly-N-isopropylacrylamide solution had a concentration of 100 mg / ml and stored it in a 4 °C refrigerator before printing to keep it in a liquid state. The nozzle spraying cell-free poly-N-isop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com