PH responsiveness supramolecular vesicle drug load system and preparation method thereof
A supramolecular, responsive technology, applied in pharmaceutical formulations, carboxylate preparation, organic active ingredients, etc., to achieve the desired effect of drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
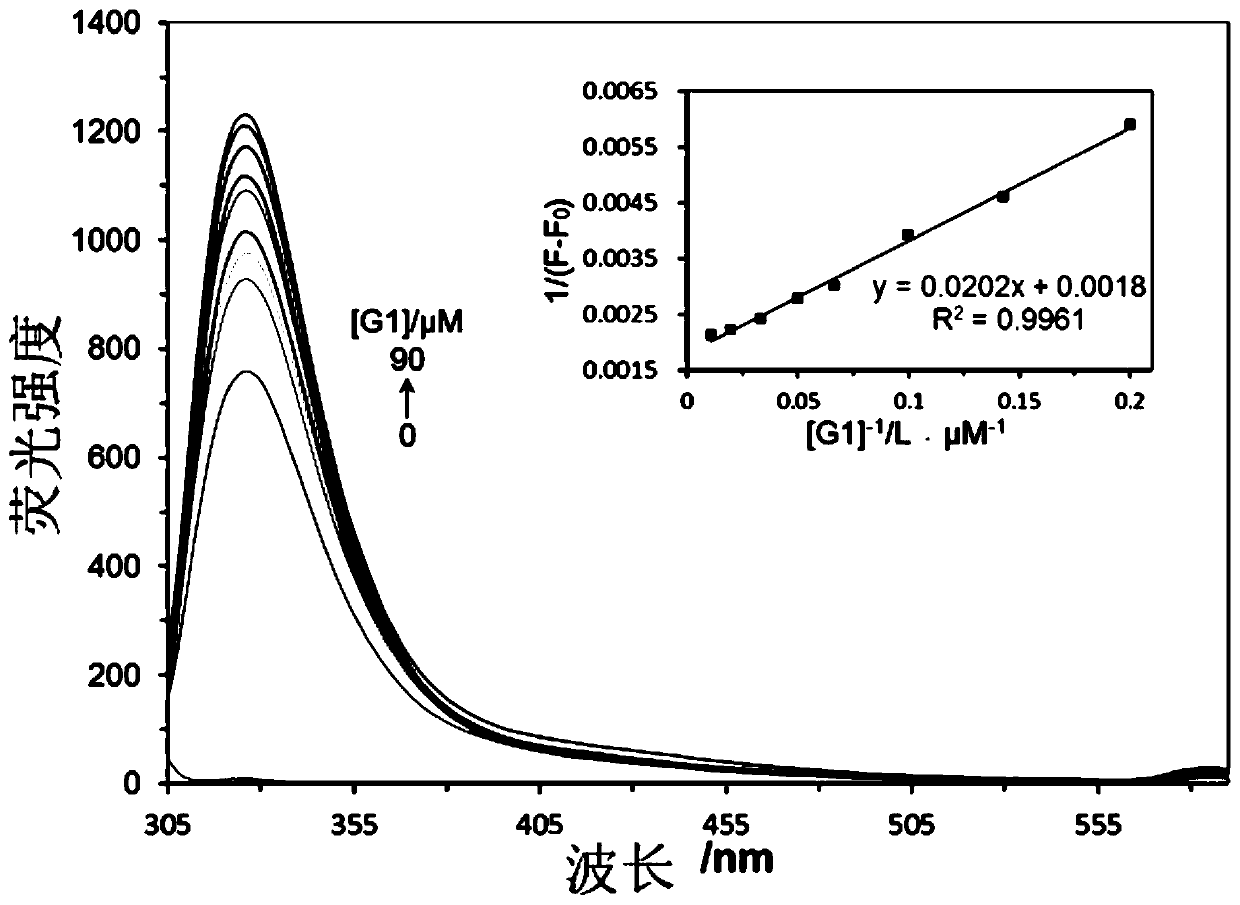
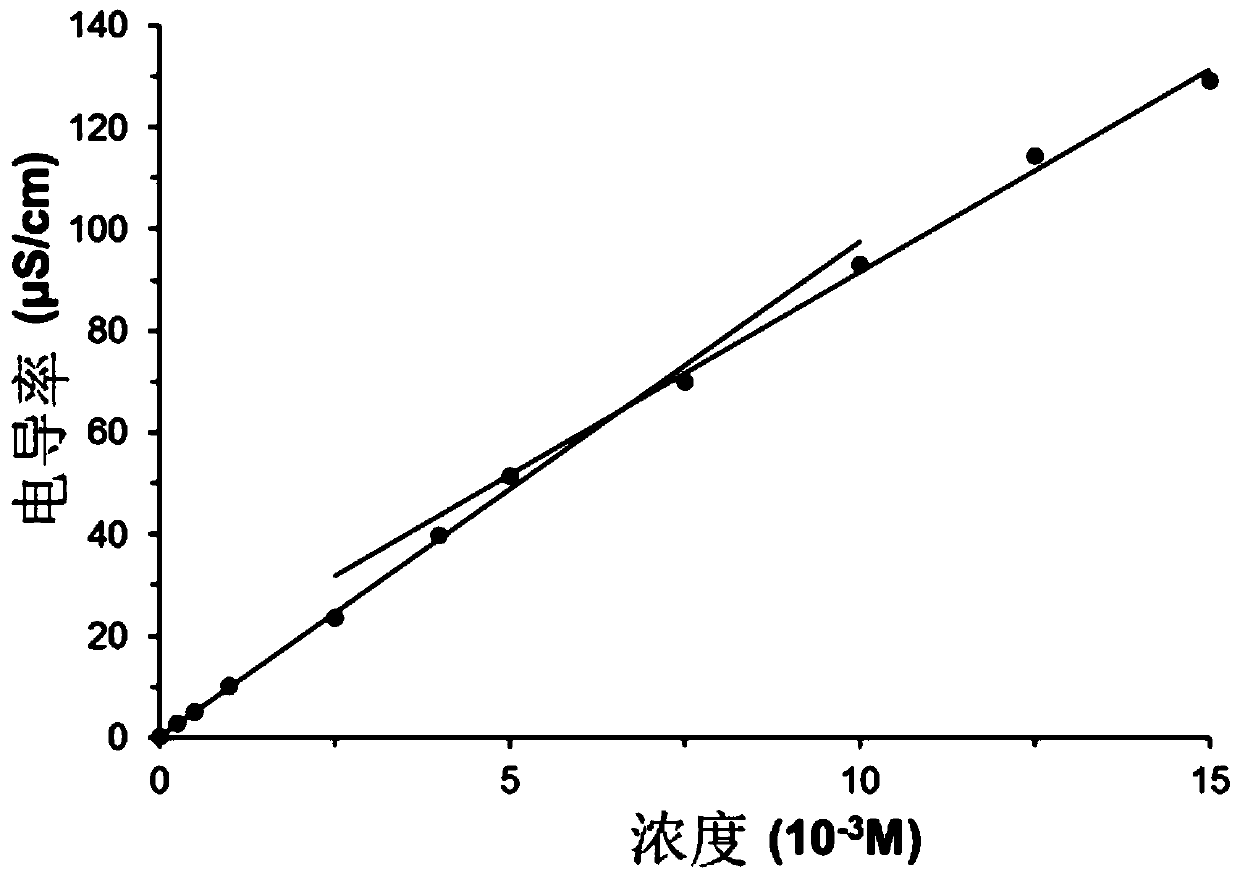
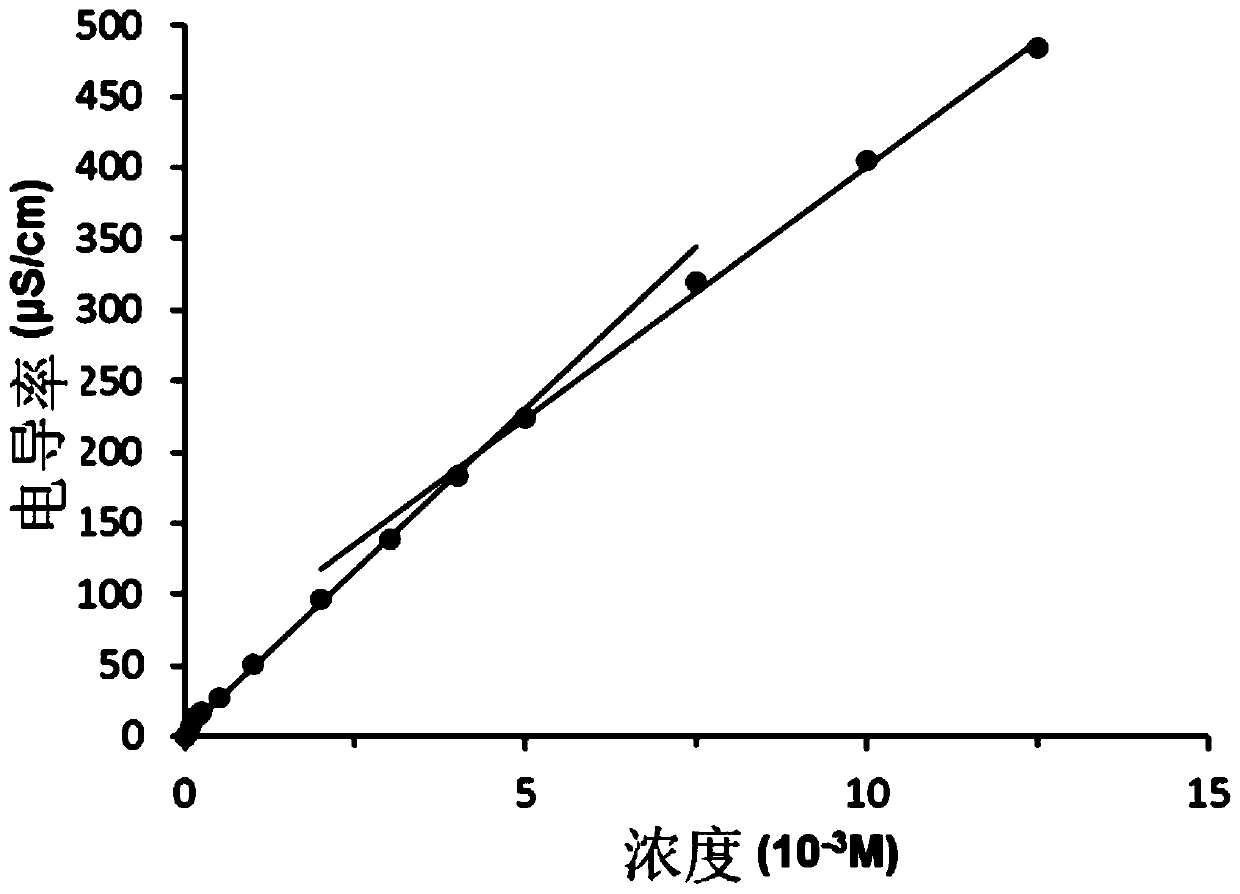
Image
Examples
Embodiment 1
[0040] (1) Synthesis of compound 2 (triphenyltripentacene hexa-substituted oxymethyl acetate)
[0041] Compound 1 (0.56mmol), potassium carbonate catalyst (10.17mmol), acetonitrile solvent (15mL) and a teaspoon of potassium iodide (catalyst) were added to a round bottom flask and stirred, under the protection of nitrogen, refluxed for 12 hours, and pumped under reduced pressure after reaction Filter to obtain a reddish-brown viscous substance, namely compound 2.
[0042] (2) Synthesis of compound 3 (triphenyltripentacene hexasubstituted oxyacetate)
[0043] Compound 2 (5.7 mmol), 40% sodium hydroxide solution (10 mL) and absolute ethanol (5.0 mL) were added into a round bottom flask and stirred, and refluxed for 12 hours under nitrogen protection. After the reaction was stopped, a brown solid, namely compound 3, was obtained after distillation under reduced pressure.
[0044] Add water to dissolve compound 3, then use HCl to adjust pH ≤ 2, compound 3 is precipitated, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com