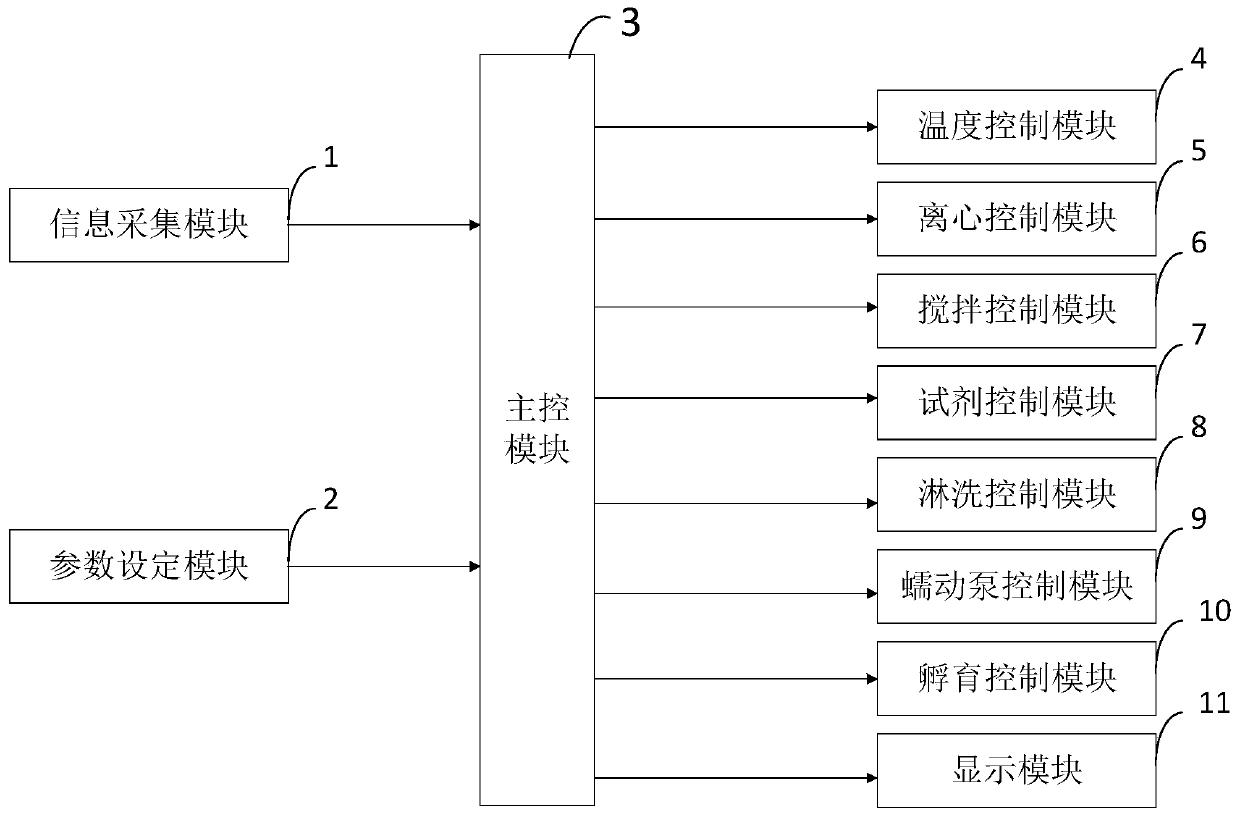
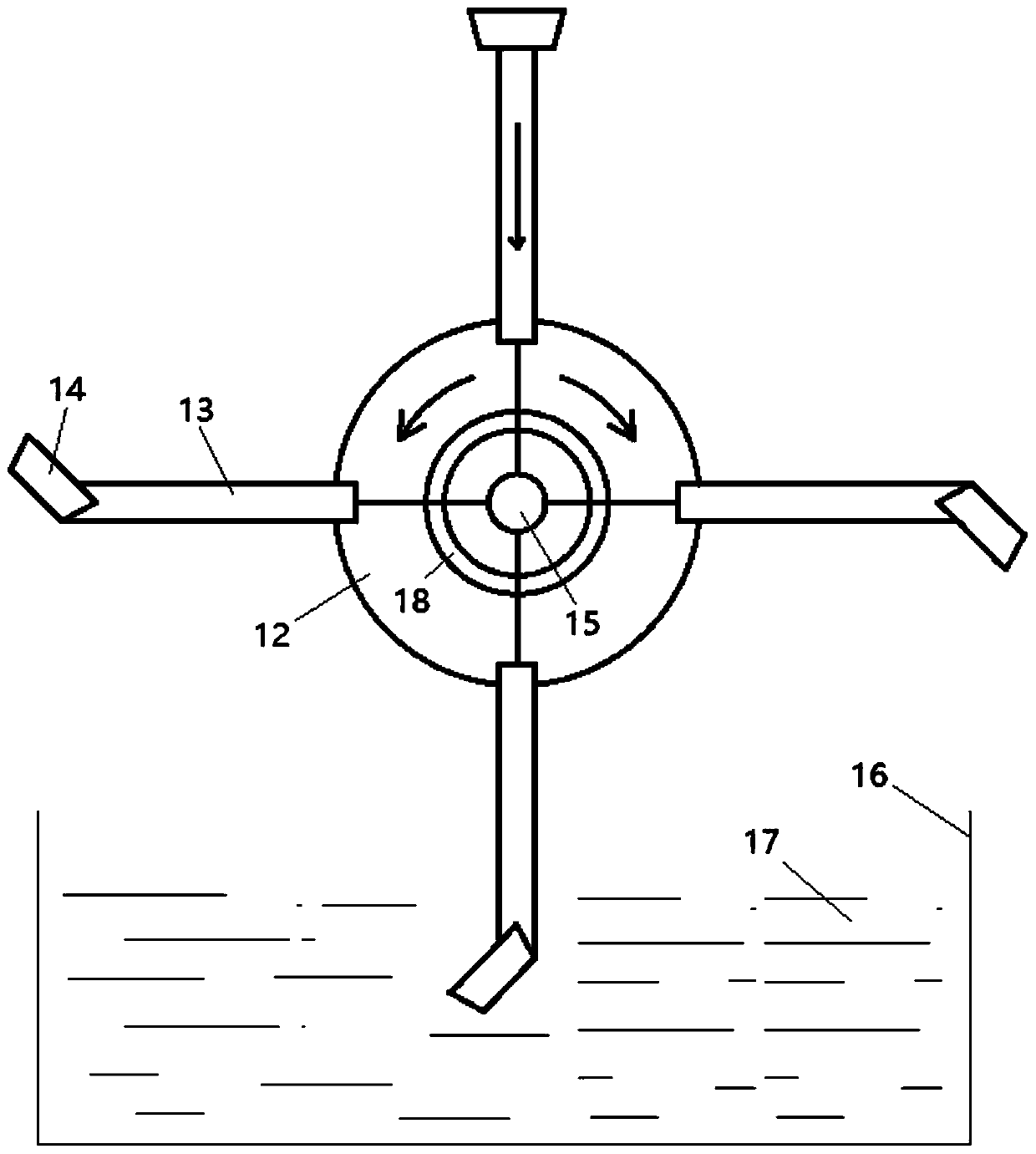
Novel erythrocyte membrane monoclonal antibody production method and processing device thereof
A technology of red blood cell membrane and production method, applied in the field of biomedicine, can solve the problems of low specificity, increased production cost, poor control accuracy of production method, etc., achieve high cancer cell uptake rate, avoid bubble generation, and sample loading effect better results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] In S109 provided by the present invention, the preparation method of the antibody nanoparticles comprises the following steps:
[0076] 1) Preparation of erythrocyte membrane: after fully anesthetizing SD rats, take blood from the heart, transfer the blood to an EP tube containing sodium heparin; centrifuge the whole blood, discard the supernatant, leave a dark red precipitate, wash with PBS, and centrifuge , and then washed several times; discard the supernatant, leave the precipitate, take PBS and blow it away, take 0.2mM EDTA and put it into a centrifuge tube to mix well, after fully hemolysis, add 20*PBS, centrifuge, and centrifuge for hemolysis several times; discard The supernatant, the precipitate is the red blood cell membrane.
[0077] 2) Preparation of antibody nanoparticles by isoelectric point precipitation: put the antibody into a dialysis bag, dialyze in ultrapure water, collect the antibody in the dialysis bag, freeze-dry, and store at -20°C; take the fre...
Embodiment 1
[0126] Example 1: Production method of novel erythrocyte membrane monoclonal antibody
[0127] 1. Workflow:
[0128] 1.1 Preparation:
[0129] 1.1.1 Reagent preparation:
[0130] Equilibration buffer: 10 mM Tris-NaCl (pH8.3).
[0131] Dissociation buffer: 0.1M citric acid (pH 4.0).
[0132] Regeneration buffer: 0.1M glycine (pH2.7).
[0133] Dialysis buffer: 20 mM Tris-NaCl (pH7.5) or 10 mM PBS (pH7.4).
[0134] pH neutralizing solution: 2M Tris (pH8.0).
[0135] 1.1.2 Equipment preparation: Protein A column, low-temperature high-speed centrifuge, balance, peristaltic pump.
[0136] 1.2 Operation steps:
[0137] 1.2.1 Sample preparation: The frozen ascites was melted at 10-15°C the day before, and filtered with absorbent cotton soaked in triple-distilled water to remove oil, and the filtered ascites was centrifuged at 4°C at 10,000 rpm for 30 minutes to collect the supernatant.
[0138]Column preparation: Fill with an appropriate amount of Protein A filler, wash with p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com