Cytoplasmic protein-assisted enhanced anthocyanin-like fluorescent dye and preparation method thereof
A cytoplasmic protein and fluorescent dye technology, which is applied in the field of synthesis and preparation of cytoplasmic protein-assisted enhanced anthocyanin-like fluorescent dyes, can solve the problems of poor stability and secondary processability, achieve enhanced fluorescence intensity, realize detection and Imaging, improve the effect of the output signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
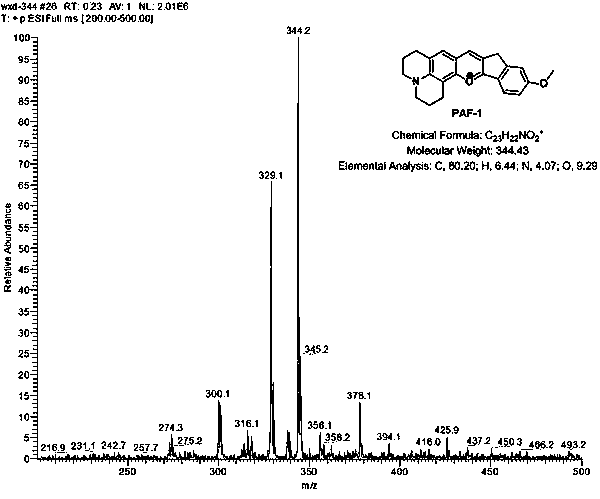
[0026] Example 1: Synthesis of n=0 anthocyanin fluorescent dye (PAF-1).
[0027] The specific synthetic route of the described class of cytoplasmic protein-assisted enhanced anthocyanin-like fluorescent dye PAF is as follows
[0028] The synthesis route of the n=0 cytoplasmic protein-assisted enhanced anthocyanin-like fluorescent dye is as follows:
[0029]
[0030] Weigh 9-formaldehyde-8-hydroxyjulolidine (217 mg, 1 mmol) and 5-methoxy-1-indanone (162 mg, 1 mmol), dissolve in 2 mL methanesulfonic acid, and Under the protection of nitrogen, react in an oil bath at 90°C for 6 hours to obtain a reaction solution. After the reactant is cooled to room temperature, slowly add the reactant dropwise to 100 g of ice water, and keep stirring. At the same time, add 1 mL of perchloric acid and stir well. , the bottom of the dye ice-water mixture was formed and there was a large amount of precipitation, extracted 4-5 times with dichloromethane-methanol (volume ratio: 25:1) mixture, 30...
Embodiment 2
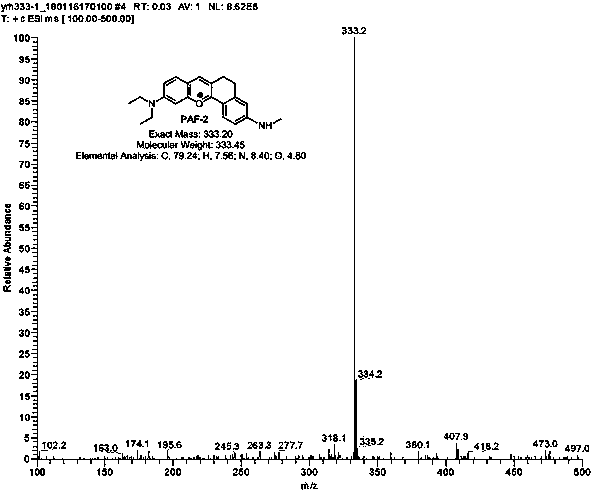
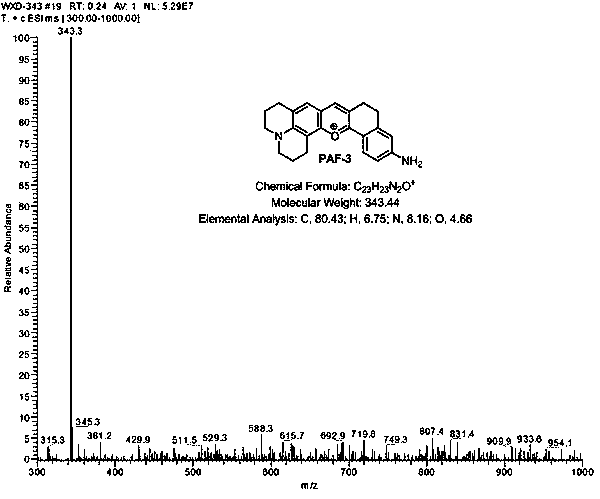
[0031] Example 2: Synthesis of anthocyanin-like fluorescent dyes (PAF-2, PAF-3, PAF-4, PAF-5) with n=1.
[0032]
[0033] Weigh the reactant reactant 1 as 4-(diethylamino) salicylaldehyde (R1 is nitrogen nitrogen diethylamino) (193 mg, 1 mmol), and reactant 6-(methylamino)-3, 4,-Dihydro-1-naphthalenone (compound 1) (175 mg, 1 mmol) was dissolved in 2 mL of methanesulfonic acid, and reacted for 6 hours under nitrogen protection, 90°C oil bath to obtain a reaction solution, which was then The reaction solution was cooled to room temperature, and the reactant was slowly added dropwise to 100 g of ice water, and kept stirring, and at the same time, 1 mL of perchloric acid was added dropwise, and the stirring was sufficient, and a large amount of precipitation was formed at the bottom of the dye ice water mixture, and dichloromethane- Methanol (volume ratio (10-30): 1) was extracted 4-5 times from the mixed solution, 30 mL each time, the extract was dried with anhydrous sodium s...
Embodiment 3
[0035] Example 3: Histogram of fluorescence intensity ratios of dyes in cell lysates and buffers of different cells.
[0036]Take 495 uL of PBS buffer (10 mM, pH=7.2) and add 5 uL of different PAF dyes respectively, and use a fluorescence spectrophotometer to detect the fluorescence intensity of different dyes. Then the buffer solution was replaced with the cell lysate of HepG-2, Hela, and MCF-7 cells respectively, and the fluorescence intensity of different dyes in the cell lysate was measured under the same conditions, and the fluorescence intensity value of the dye in the cell lysate was Compared with the fluorescence intensity value in PBS buffer, get as image 3 The histogram shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com