Porous polyaryletherketone microsphere and preparation method thereof
A technology of porous microspheres and polyaryletherketone, which is applied in the field of porous materials, can solve the problems of subsequent application influence, residual porogen, complicated process, etc., and achieve the effects of improving purity, simple preparation process and low energy consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
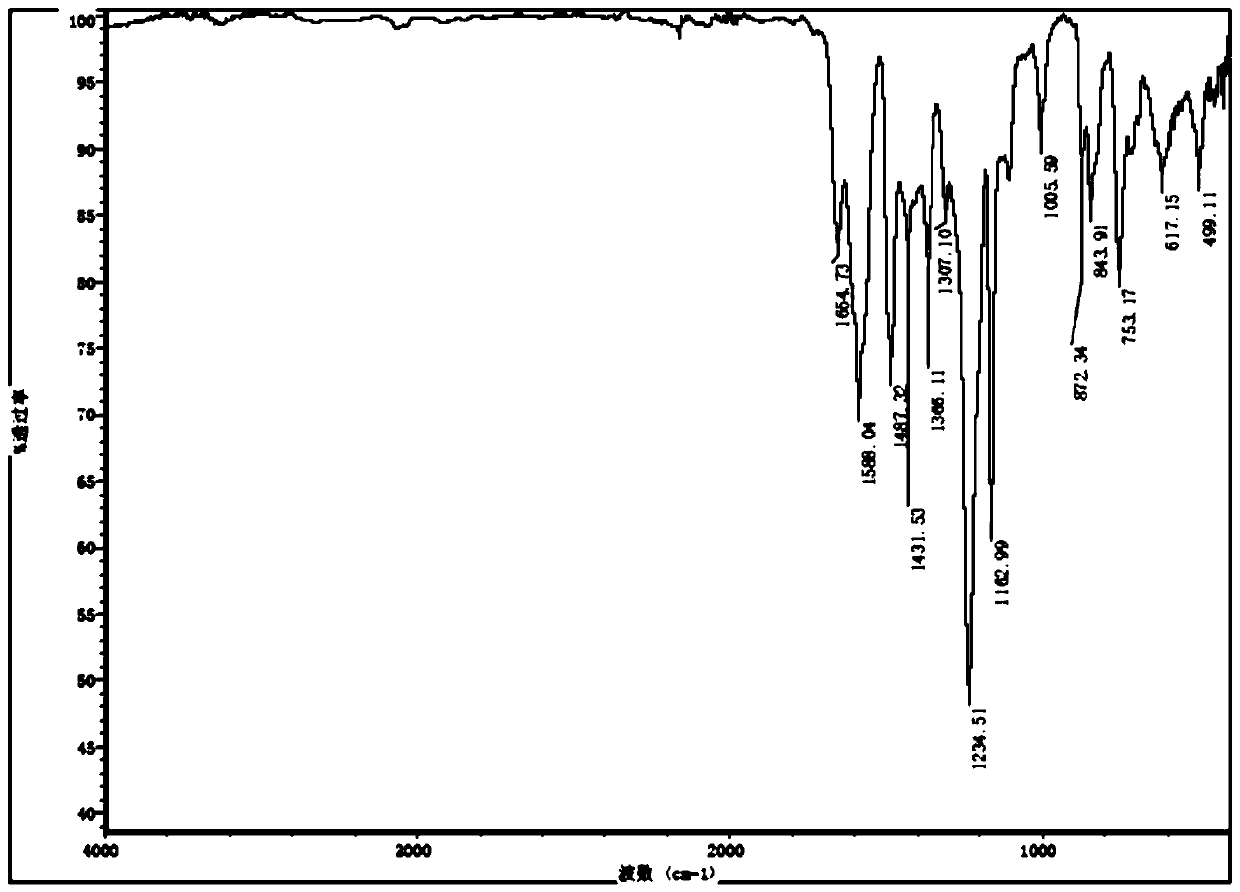
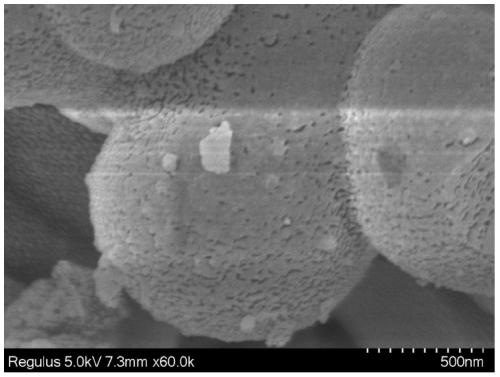
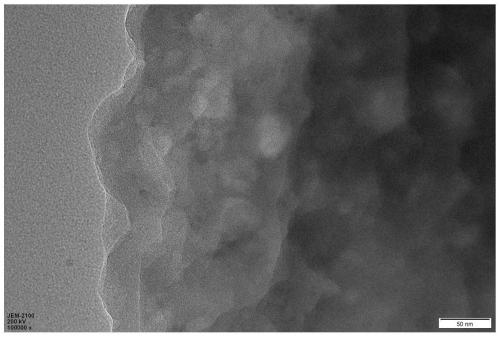
[0034] Quickly weigh 0.500 g of 1,3,5-benzenetricarboxylic acid chloride and 0.481 g of diphenyl ether into a 250 mL three-necked flask, and then add 100 mL of dichloromethane. Quickly weigh 5.359g of titanium tetrachloride and add it into a three-necked flask, and shake it with a shaker for about 1min. After standing at room temperature for 72 hours, the orange-red transparent liquid gradually became turbid and precipitated out. The reaction mixture was placed in a centrifuge tube for separation under the conditions of 10000 rpm and 1 min. The obtained solid was washed with dichloromethane and centrifuged three times, and the solid in the centrifuge tube was put into a drying oven to dry overnight to finally obtain a dark yellow solid powder, namely polyaryletherketone porous microspheres.
Embodiment 2
[0036] Quickly weigh 0.700 g of 1,3,5-benzenetricarboxylic acid chloride and 0.481 g of diphenyl ether into a 250 mL three-necked flask, and then add 100 mL of chloroform. Quickly weigh 5.359g of titanium tetrachloride and add it into a three-necked flask, and shake it with a shaker for about 1min. After standing at room temperature for 12 hours, the orange-red transparent liquid gradually became turbid and precipitated out. The reaction mixture was placed in a centrifuge tube for separation under the conditions of 10000 rpm and 1 min. The obtained solid was washed with acetone and centrifuged three times, and the solid in the centrifuge tube was put into a drying oven to dry overnight, and finally dark yellow solid powder was obtained, namely polyaryletherketone porous microspheres.
Embodiment 3
[0038] Quickly weigh 0.800 g of 1,3,5-benzenetricarbonyl chloride and 0.481 g of diphenyl ether into a 250 mL three-necked flask, and then add 100 mL of dimethyl sulfoxide. Quickly weigh 5.359g of titanium tetrachloride and add it into a three-necked flask, and shake it with a shaker for about 1min. After standing at room temperature for 24 hours, the transparent liquid gradually became cloudy and precipitated out. The reaction mixture was placed in a centrifuge tube for separation under the conditions of 10000 rpm and 1 min. The obtained solid was washed with acetone and centrifuged three times, and the solid in the centrifuge tube was put into a drying oven to dry overnight, and finally dark yellow solid powder was obtained, namely polyaryletherketone porous microspheres.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com