Helicobacter pylori CagL protein detection kit and detection method thereof
A technology for detection of Helicobacter pylori and protein, which is applied in the field of medicine and biology, can solve problems such as difficult acceptance by patients and family members, and great pain for patients, and achieve the effects of rapid diagnosis, convenient operation, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
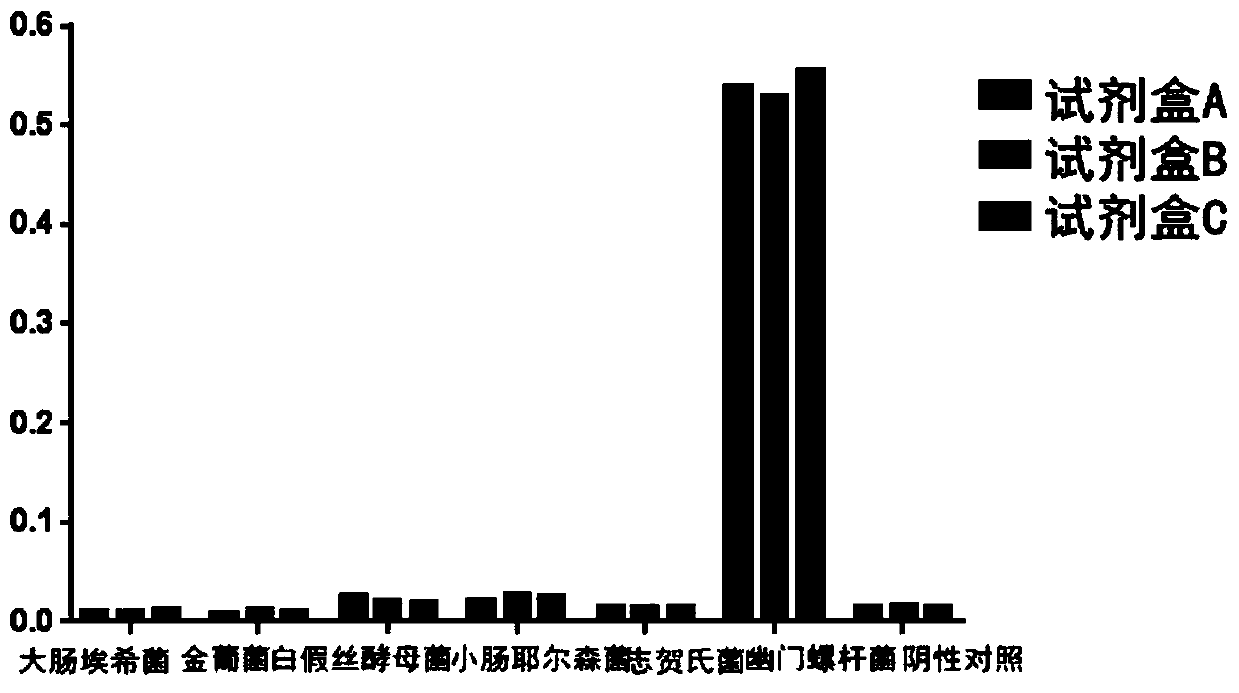
[0043] Helicobacter pylori CagL protein detection kit, coated with mouse monoclonal CagL antibody on a microwell plate, combined with rabbit polyclonal CagL antibody and HRP-labeled goat anti-rabbit IgG antibody, using the principle of double antibody sandwich method for detection CagL protein in samples.
[0044] in:
[0045] The preparation method of rabbit polyclonal CagL antibody is as follows: New Zealand white rabbits are used for subcutaneous injection, and the CagL protein of 0.5 mg / ml is mixed with an equal volume of complete Freund's adjuvant for the first immunization, and the back is subcutaneously injected at multiple points, each 2ml; 14 days later. For the second immunization, take 0.5mg / ml CagL protein mixed with incomplete Freund's adjuvant, and inject subcutaneously on the back, 2ml each; 21 days later, carry out the third immunization, the method is the same as the second immunization, but reduce the injection volume, 1ml each; After 28 days of booster immu...
Embodiment 2
[0066] Based on the kit of Example 1, as a further improvement to the present invention, the detection method of the kit is provided:
[0067] The detection method of the kit comprises the following steps:
[0068] 1) Coating: Dilute the CagL monoclonal antibody to 1:32000 with coating buffer, add 100ul per well to a 96-well microplate, overnight at 4°C, wash the plate 3 times with PBST for 5min each time, and finally shoot Dry;
[0069] 2) Blocking: add 5% skimmed milk powder, 200 μL per well, and incubate at 37°C for 2 hours;
[0070] 3) Washing: Discard the blocking solution, wash the plate 3 times with PBST buffer, each time for 5 minutes, and finally pat dry;
[0071] 4) Add the sample to be tested: add 100ul of the sample to be tested to each well, and add 100ul each of the negative control substance and the positive control substance at the same time, and incubate at 37°C for 1h;
[0072] 5) Washing: Discard the liquid, wash the plate 3 times with PBST buffer, each t...
Embodiment 3
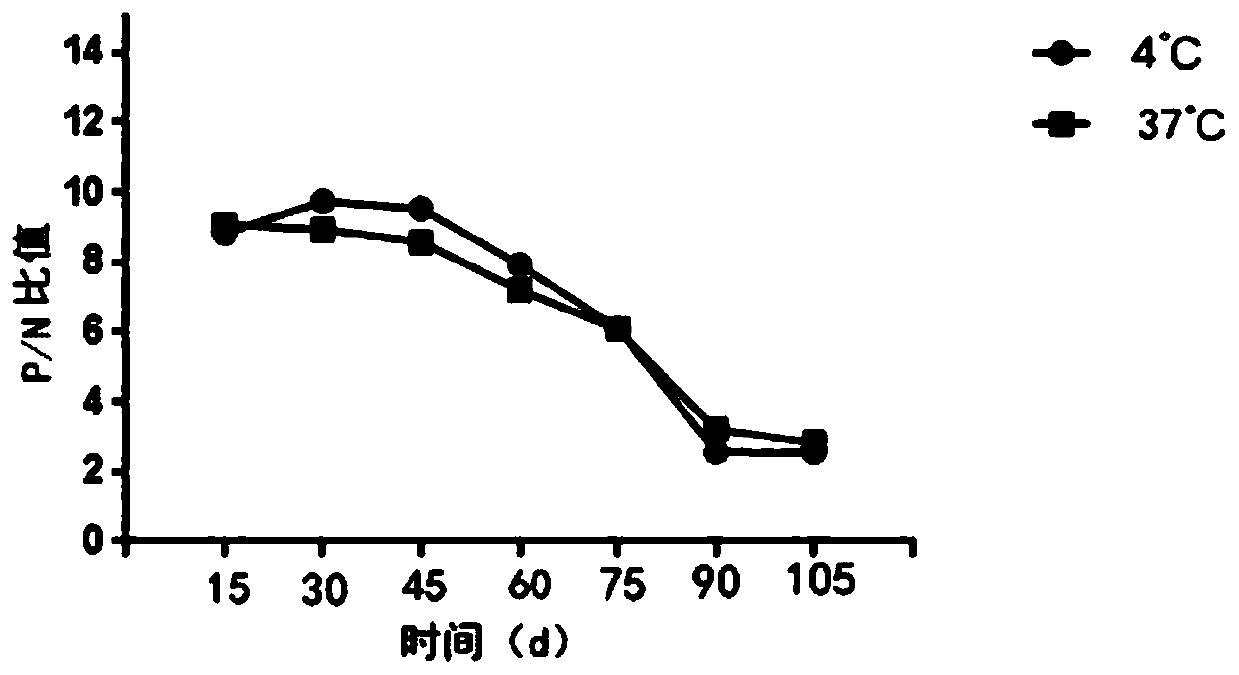
[0083] Based on the detection method in Embodiment 2, in this embodiment, the detection sample of the kit is Helicobacter pylori, and the OD value at a wavelength of 450 / 620 nm is measured by a microplate reader.
[0084] The judgment method is: OD4 detected by the sample 50 / 620nm When the value>0.0536, it can be judged as positive.
[0085] The preparation method is as follows: select well-grown Helicobacter pylori strains, add physiological saline to adjust to suspend bacteria liquid, and adjust the minimum to OD of 1.0.
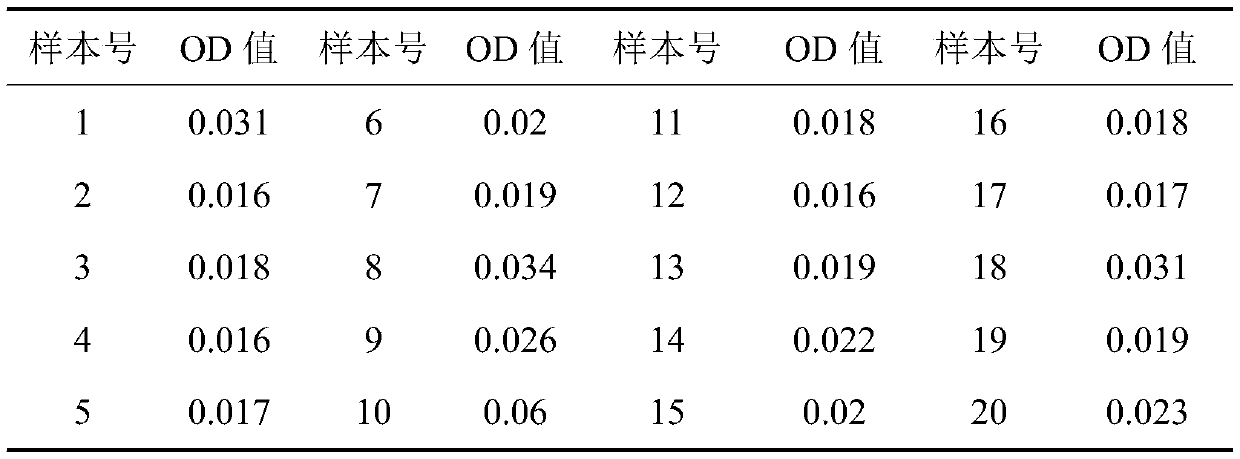
[0086] The detection values of 20 negative samples are shown in the table below:
[0087]
[0088] Detection value of different concentrations of bacterial liquid
[0089]
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com