Fluorescent probe for detecting mercury ions as well as preparation method and use method thereof
A fluorescent probe, mercury ion technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of unfavorable and accurate detection, quantitative detection of samples that are difficult to contain mercury ions, detection limit, etc. Application, easy operation, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
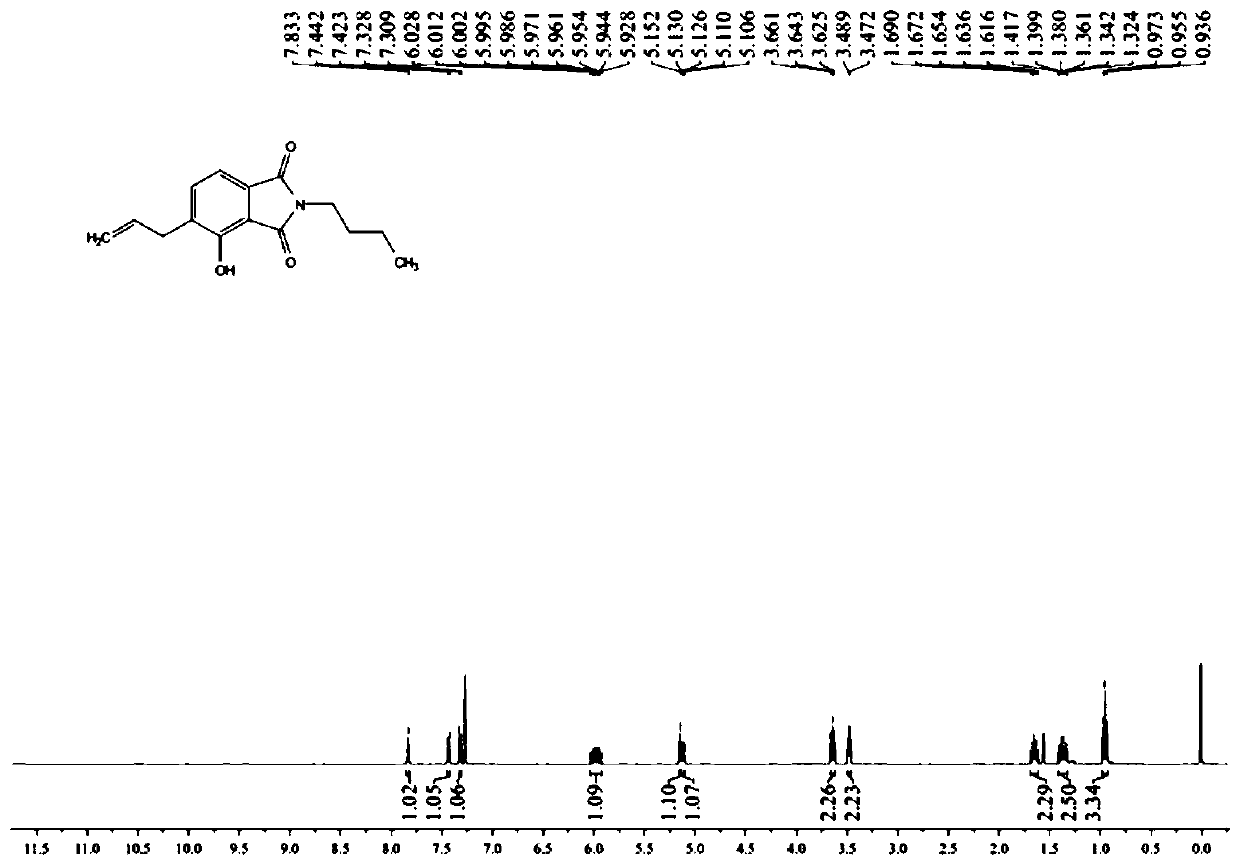
[0038] Embodiment 1, the synthesis of compound NMS1
[0039] Synthesis of N-n-butyl-3-(2'-bromoethyl)-oxyphthalimide (4)
[0040] 1.32g N-n-butyl-3-hydroxyphthalimide (1) (6.03mmol), 0.54mL allyl bromide (2) (6.03mmol), and 1.2g potassium carbonate (3) ( 9.03mmol) was dissolved in 50mL acetone, and after reflux stirring for 12 hours, 1.4g of compound N-n-butyl-3-(2'-bromoethyl)-oxyphthalimide (4) was obtained, producing The rate is 92%.
[0041] 1 H NMR (400MHz, Chloroform-d) δ7.62 (dd, J = 8.4, 7.3Hz, 1H), 7.43 (d, J = 7.3Hz, 1H), 7.18 (d, J = 8.4Hz, 1H), 6.09 (ddt, J=17.2,10.2,5.0Hz,1H),5.54(dd,J=17.3,1.4Hz,1H),5.36(dd,J=10.6,1.4Hz,1H),4.79(dt,J=4.9 ,1.5Hz,2H),3.66(t,J=7.2Hz,2H),1.66(q,J=7.4Hz,2H),1.36(dq,J=14.7,7.4Hz,2H),0.94(t,J =7.4Hz, 3H).
[0042] Synthesis of Compound N-n-Butyl-3-Hydroxy-4-allylphthalimide (5)
[0043] Add 1.5g of N-n-butyl-3-(2'-bromoethyl)-oxyphthalimide (4) (5.8mmol) to 4mL o-dichlorobenzene, microwave at 200 degrees After reacting for 1 hou...
Embodiment 2
[0045] Embodiment 2, the color response of compound NMS1 to mercury ion
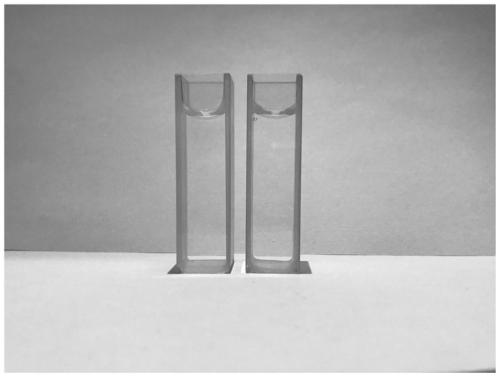
[0046] A test mother solution of dimethyl sulfoxide (DMSO) of the fluorescent probe NMS1 for detecting mercury ions according to the present invention with a concentration of 1 mM was prepared for use. Measure 10 μL of this mother solution and add it dropwise to a phosphate buffer solution with a certain concentration of mercury ions, and use the corresponding phosphate buffer solution to adjust the volume to 10 mL, so that in the test solution, the concentration of the probe is 1.0 μM, and the concentration of mercury ions is 10.0 μM for color response testing. like figure 2 and 3 As shown, after the mercury ion aqueous solution was added, the color of the solution was observed to change from light yellow to colorless, and the fluorescence of the solution also changed from almost green fluorescence to blue fluorescence, indicating that the probe NMS1 has an intuitive color development for mercury ion...
Embodiment 3
[0047] Embodiment 3, the ultraviolet titration detection of different concentrations of mercury ions to compound NMS1
[0048] A test mother solution of dimethyl sulfoxide (DMSO) of the fluorescent probe NMS1 for detecting mercury ions according to the present invention with a concentration of 1 mM was prepared for use. Measure 10 μL of this mother solution and add it dropwise to phosphate buffer with different concentrations of mercury ions, and use the corresponding phosphate buffer to make up to 10 mL, so that in the test solution, the concentration of the probe is 1.0 μM, and the concentration of mercury ions is 0-40.0μM for absorption spectrum test. The ultraviolet absorption curves in each system were obtained, and the standard curves of absorbance and mercury ion concentration were established. like Figure 4 As shown, with the increase of mercury ion concentration, the absorbance at 514nm decreases gradually and the absorbance at 427nm increases gradually, and their ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com