Dipicolinamide compound and synthetic method thereof
A technology of bispyridine amide and synthesis method, which is applied in chemical instruments and methods, water treatment of special compounds, organic compound/hydride/coordination complex catalyst, etc., can solve problems such as pH partial acidity, and achieve mild reaction conditions, The effect of high reaction efficiency and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
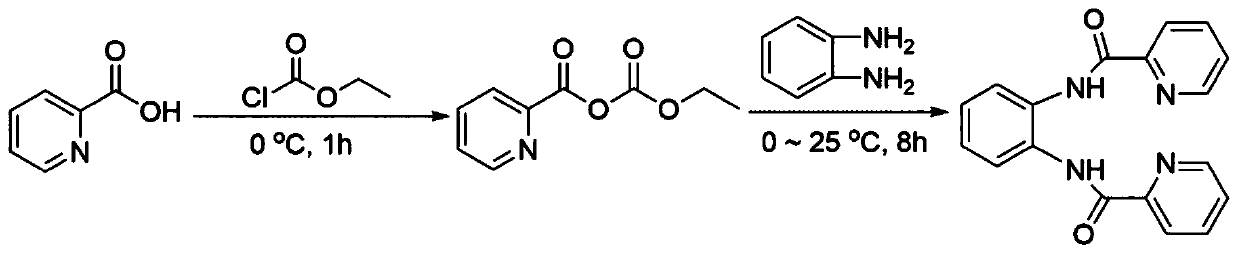
[0021] Specific embodiment one: The structural formula of the bispyridine amide compound in this embodiment is as follows:
[0022]
specific Embodiment approach 2
[0023] Specific embodiment two: the synthetic method of bispyridine amide compound described in specific embodiment one is carried out according to the following steps:
[0024] 1. Dissolve 1.0-1.4g of 2-pyridinecarboxylic acid in 25-55mL of anhydrous tetrahydrofuran, add 0.9-1.2g of triethylamine dropwise, and add 0.95-1.25g of ethyl chloroformate dropwise under nitrogen protection, and then The reaction was stirred for 40 to 80 minutes under the condition of an ice-water bath to obtain ethyl 2-pyridine anhydride;
[0025] 2. Add 0.45-0.55g of o-phenylenediamine to ethyl 2-pyridine anhydride, stir at room temperature for 6-10 hours, add 45-55mL of ethyl acetate, wash three times, dry, spin to dry the solvent, and apply the sample by dry method. Separation by silica gel column chromatography to obtain a bispyridine amide compound (N,N'-phthalic bis-(2-pyridinecarboxamide)-amine).
specific Embodiment approach 3
[0026] Embodiment 3: This embodiment is different from Embodiment 2 in that in step 1, the reaction is stirred and reacted in an ice-water bath for 50 to 70 minutes. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com