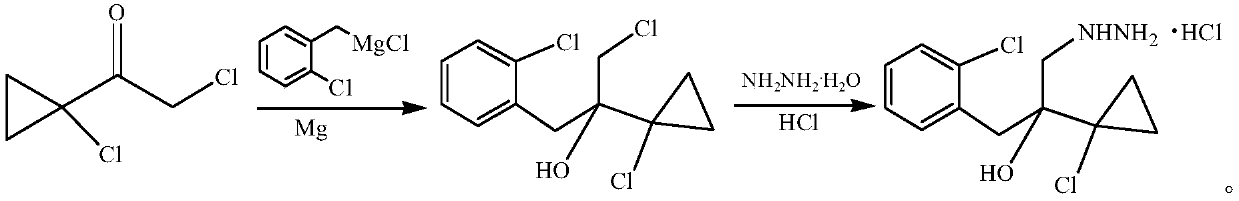
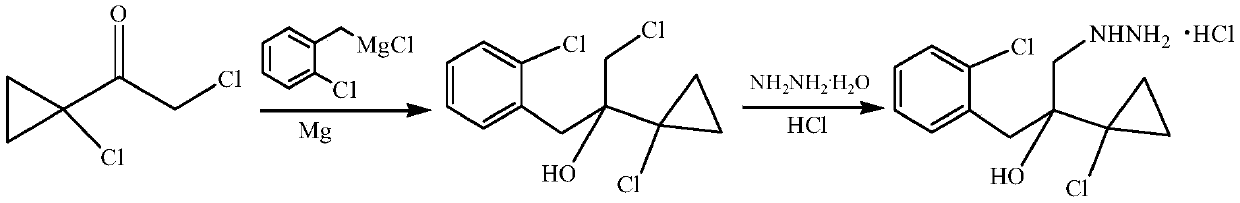
Synthetic method of 1-(2-chlorophenyl)-2-(1-chlorocyclopropyl)-3-hydrazino-2-propanol hydrochloride
A technology of propanol hydrochloride and chlorocyclopropyl, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of hydrazine, etc., can solve the problems of long reaction time, content reduction, compound instability, etc., and achieve short reaction time , Solve the effects of poor stability and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] First prepare toluene (6.5L) / 2-methyltetrahydrofuran (2.5L) mixed solvent for later use. The 50L reactor was evacuated and replaced with nitrogen twice, then at 25°C, 465g (19.4mol) of magnesium powder, 2.5g (0.01mol) of iodine, 0.81L of mixed solvent and 90g (0.056mol) of o-chlorobenzyl chloride were added, Stir at low speed until the color of iodine disappears and a little boiling occurs, that is, the reaction is initiated. Mix the remaining mixed solvent with 2.91kg (18.07mol) o-chlorobenzyl chloride, add dropwise, and finish dropping in about 4 hours. The reaction was continued at 50°C for 0.5h. Cool down to 0-5°C, add 2.64 kg (17.26 mol) of 1-chloro-1-acetylcyclopropane dropwise, and continue the reaction for 1 h after the drop is complete. Add 5% dilute hydrochloric acid dropwise to pH = 7, and separate the layers. The organic phase was washed twice with water, separated, and the organic phase was precipitated to obtain 5.07 kg of crude Grignard product. Based...
Embodiment 2
[0022] First prepare tert-butyl methyl ether (6.9L) / 2-methyltetrahydrofuran (2.3L) mixed solvent for later use. Vacuumize the 50L reactor and replace it with nitrogen twice, then add 456g (19mol) magnesium powder, 2.5g (0.01mol) iodine, 0.9L mixed solvent and 90g (0.056mol) o-chlorobenzyl chloride at 25°C, Under stirring, until the color of iodine disappears, a little boiling occurs, that is, the reaction is initiated. Mix the remaining mixed solvent with 2.91kg (18.07mol) o-chlorobenzyl chloride, add dropwise, and finish dropping in about 4 hours. The reaction was continued at 50°C for 0.5h. Cool down to 0-5°C, add 2.64 kg (17.26 mol) of 1-chloro-1-acetylcyclopropane dropwise, and continue the reaction for 1 h after the drop is complete. Add 5% dilute hydrochloric acid dropwise to pH = 7, and separate the layers. The organic phase was washed twice with water, separated, and the organic phase was precipitated to obtain 5.18 kg of crude Grignard product. Based on 1-chloro-1...
Embodiment 3
[0025] First prepare toluene (8.1L) / 2-methyltetrahydrofuran (1.8L) mixed solvent for later use. Vacuumize the 50L reactor and replace it with nitrogen twice, then add 456g (19mol) magnesium powder, 2.5g (0.01mol) iodine, 1000mL mixed solvent and 90g (0.056mol) o-chlorobenzyl chloride at 25°C, and stir at a low speed Next, when the color of iodine disappears and a little boiling occurs, the reaction starts. Mix the remaining mixed solvent with 2.91kg (18.07mol) o-chlorobenzyl chloride, add dropwise, and finish dropping in about 5 hours. The reaction was continued at 50°C for 0.5h. Cool down to 0-5°C, add 2.59 kg (16.94 mol) of 1-chloro-1-acetylcyclopropane dropwise, and continue the reaction for 1 h after the drop is complete. Add 5% dilute hydrochloric acid dropwise to pH = 7, and separate the layers. The organic phase was washed twice with water, separated, and the organic phase was precipitated to obtain 5.97 kg of crude Grignard product. Based on 1-chloro-1-acetylcyclop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com