Preparation method of high molecular weight branched polymers
A branched polymer, high molecular weight technology, applied in the field of preparation of high molecular weight branched polymers, can solve the problems of limited types of thiol monomers, limited molecular weight of polymers, easy cross-linking of polymers, etc., and achieves a controllable structure. , The effect of simple components and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
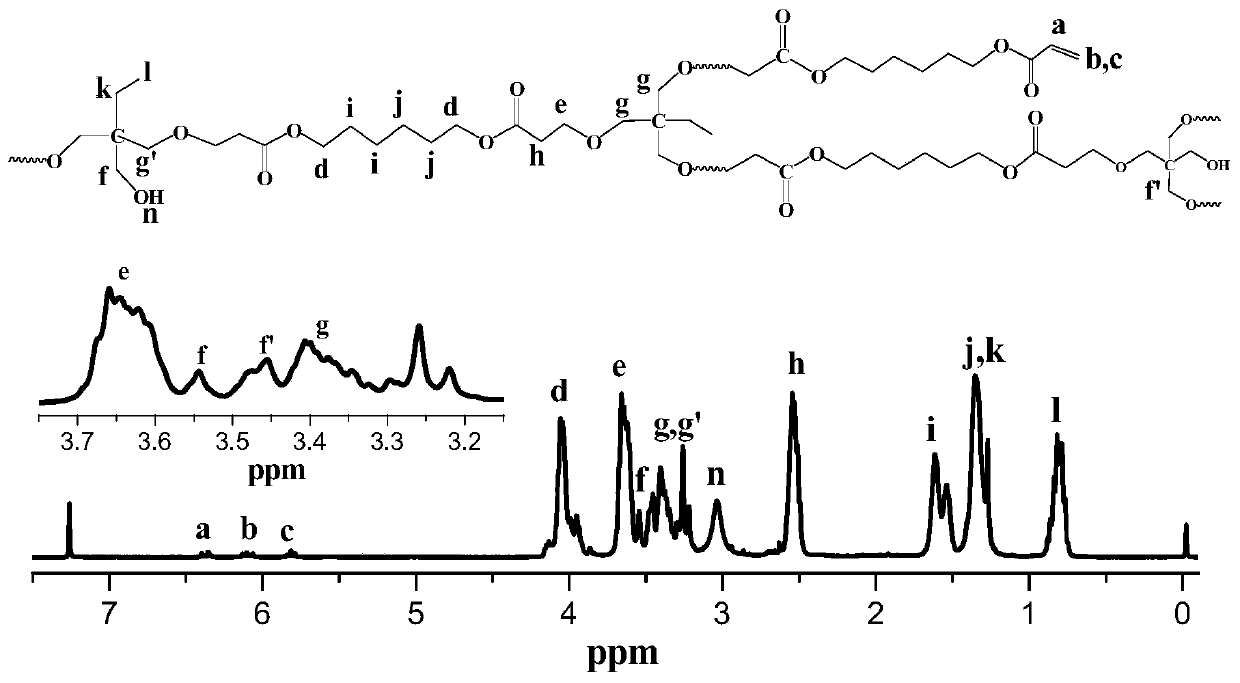
[0025] Trimethylolpropane (0.134 g, 1 mmol) and 1,6-hexanediol diacrylate (0.226 g, 1 mmol) dissolved in DMF (1.217 g) were added to a dehydrated and argon-purged polymerization bottle , after freezing and evacuating argon for three times, under the protection of argon, use a 100 μL micro-injector to add the catalyst t-BuP at a rate of 3-5 s per drop 2 (50 μL, 0.1 mmol) was reacted at 25° C. for 60 min. After the reaction was completed, acetic acid was added to terminate the reaction, diluted with dichloromethane, and settled in n-hexane through an acidic alumina column to obtain a hydroxyl-terminated polymer with a yield of 52.5%. Adopt nuclear magnetic resonance spectrometer and gel permeation chromatography to analyze polymer, the result is as follows: Gel permeation chromatography relative weight-average molecular weight M w.GPC =6461g / mol, molecular weight distribution PDI=1.90, absolute weight average molecular weight M w.MALLS =33080g / mol, the branching degree DB calc...
Embodiment 2
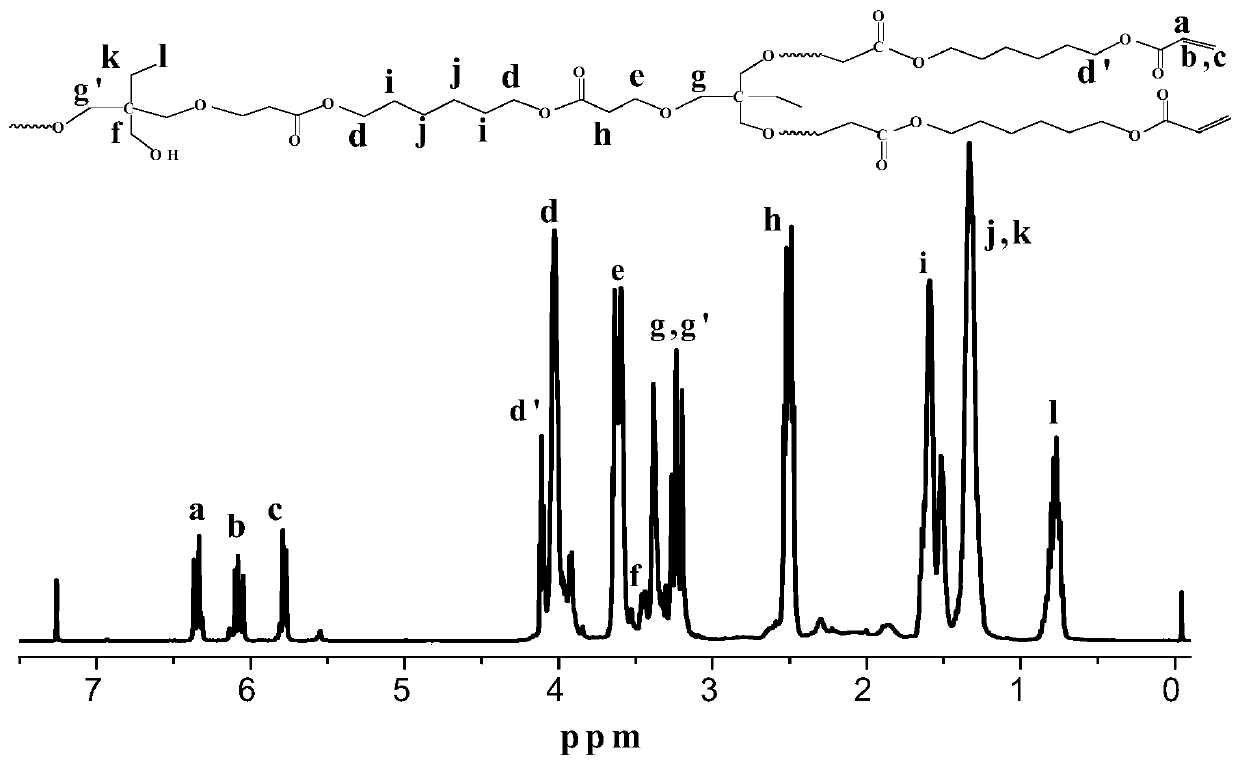
[0027] Trimethylolpropane (0.134 g, 1 mmol) and 1,6-hexanediol diacrylate (0.226 g, 1.5 mmol) dissolved in DMF (1.217 g) were added to a dehydrated and argon-purged polymerization bottle In the process, after freezing and evacuating argon for three times, under the protection of argon, add the catalyst t-BuP with a 100 μL micro-injector at a rate of 3-5 s per drop. 2 (50 μL, 0.1 mmol) was reacted at 25° C. for 60 min. After the reaction was completed, acetic acid was added to terminate the reaction, dichloromethane was diluted, and the acidic alumina column was settled in n-hexane to obtain a polymer containing hydroxyl groups and double bond ends with a yield of 58.6%. Adopt nuclear magnetic resonance spectrometer and gel permeation chromatography to analyze polymer, the result is as follows: Gel permeation chromatography relative weight-average molecular weight M w.GPC =7002g / mol, molecular weight distribution PDI=2.59, absolute weight average molecular weight M w.MALLS =5...
Embodiment 3
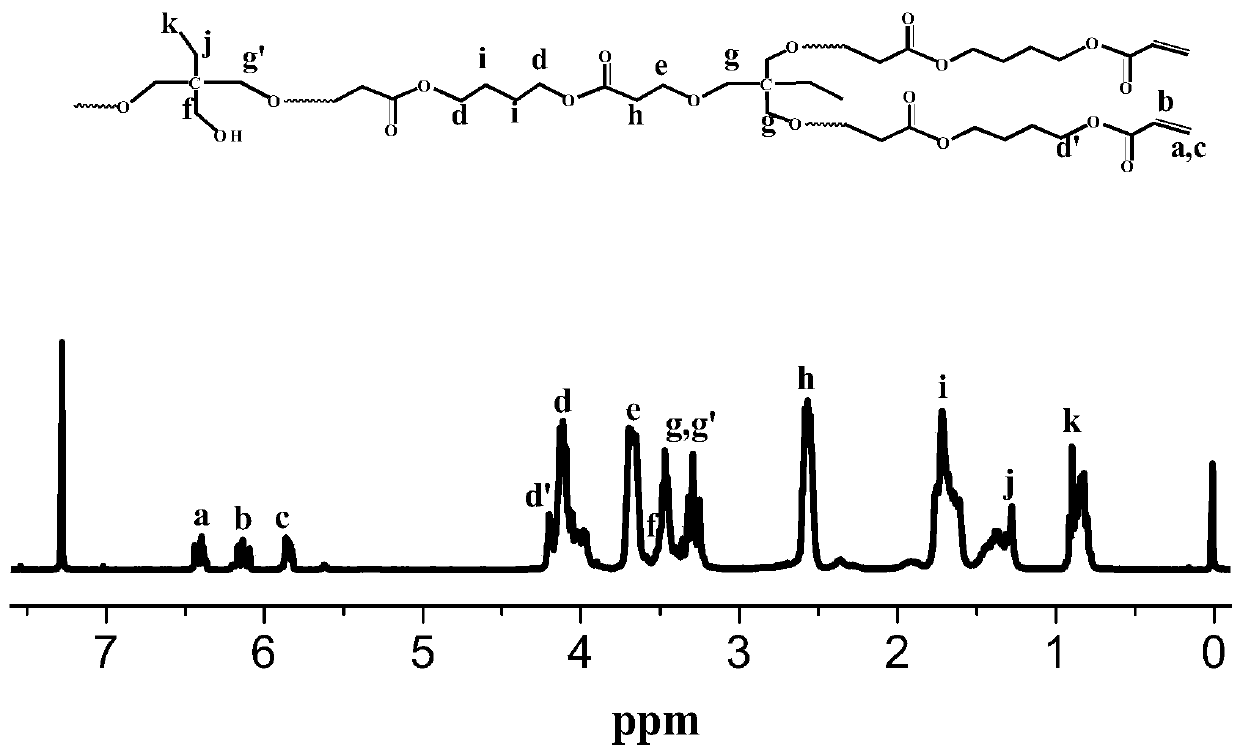
[0029] Trimethylolpropane (0.134 g, 1 mmol) and 1,6-hexanediol diacrylate (0.452 g, 2 mmol) dissolved in DMF (1.172 g) were added to a dehydrated and argon-purged polymerization bottle , after freezing and evacuating argon for three times, under the protection of argon, use a 100 μL micro-injector to add the catalyst t-BuP at a rate of 3-5 s per drop 2 (25 μL, 0.05 mmol) was reacted at 25° C. for 60 min. After the reaction was completed, acetic acid was added to terminate the reaction, diluted with dichloromethane, and settled in n-hexane through an acidic alumina column to obtain a double-bond-terminated polymer with a yield of 45.8%. Polymer is analyzed by proton nuclear magnetic resonance spectrometer and gel permeation chromatography, and the results are as follows: Gel permeation chromatography relative weight average molecular weight M w.GPC =4468g / mol, molecular weight distribution PDI=2.30, absolute weight average molecular weight M w.MALLS =21960g / mol, the branching...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com