Method for detecting trinitride compounds in candesartan cilexetil
A technology of candesartan cilexetil and azide compounds, which is applied in the field of detection of azide compounds in candesartan cilexetil, can solve the problems of time-consuming and cumbersome steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the HPLC detection of candesartan cilexetil bulk drug
[0032] Adopt detection method of the present invention, specific implementation is as follows:
[0033] Instrument: Waters e2695-2489 high performance liquid chromatography
[0034] Chromatographic column: Octadecylsilane bonded silica gel as filler (Waters Atlantis T3 C18 4.6*250mm, 5μm)
[0035] Mobile phase A: The volume ratio of acetonitrile and sulfuric acid aqueous solution is 40:60 as mobile phase A.
[0036] Mobile phase B: The volume ratio of acetonitrile to water is 90:10 as mobile phase B.
[0037] Carry out gradient elution according to the following table 1:
[0038] Table 1 Elution program
[0039]
[0040] Column temperature: 30°C
[0041] Flow rate: 0.7ml / min
[0042] Injection volume: 50μl
[0043] Detection wavelength: 205nm
[0044] Workstation: Empower 3
[0045] Test solution: Accurately weigh about 80 mg of candesartan cilexetil raw material drug to be tested, put it ...
Embodiment 2
[0050] Embodiment 2: Determination of detection wavelength
[0051] Instrument: UV-Vis spectrophotometer
[0052] Detection wavelength: 200-400nm full wavelength scanning
[0053] Accurately weigh about 30 mg of sodium azide reference substance, put it in a 250ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and use it as the reference substance mother solution. Precisely measure 5.0ml of the mother solution of the reference substance, put it in a 10ml measuring bottle, add diluent to the mark, shake well, and use it as the reference substance solution for wavelength detection.
[0054] Take an appropriate amount of sodium azide wavelength detection reference substance solution, measure according to the "UV-Vis Spectrophotometry Operating Procedures", record the spectral scanning curve, and see the attached figure 1 .
[0055] Refer to attached figure 1 , from the results of the ultraviolet scanning diagram: sodium azide has a relatively lar...
Embodiment 3
[0056]Embodiment 3: System suitability and specificity test
[0057] The system applicability of this embodiment also examines the system applicability of the instrument during continuous sample injection.
[0058] Blank solution: diluent;
[0059] Reference substance solution, system suitability solution: the preparation method is the same as in Example 1;
[0060] According to the chromatographic conditions in Example 1, 1 injection of blank solution, 6 injections of reference solution, and 1 injection of system suitability solution were sequentially injected, and the relative standard deviation of retention time and peak area was calculated, and the results are shown in Table 2.
[0061] Table 2 System Suitability Results
[0062]
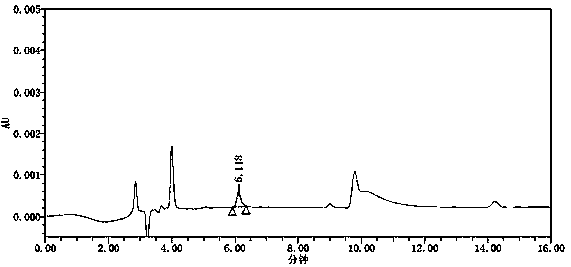
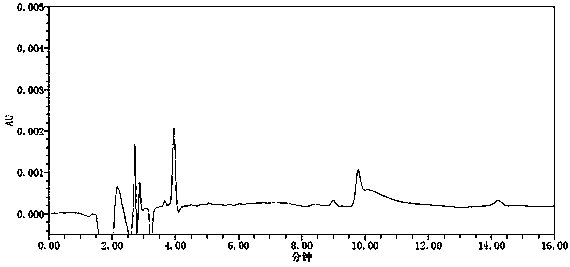
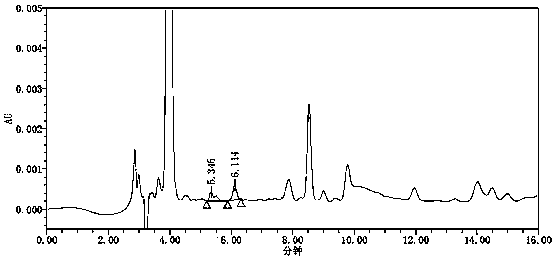
[0063] Conclusion: see attached figure 2 , the blank solution has no interference. Refer to attached image 3 , in the chromatogram obtained from the reference substance solution, the signal-to-noise ratio of the azide peak is above 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com