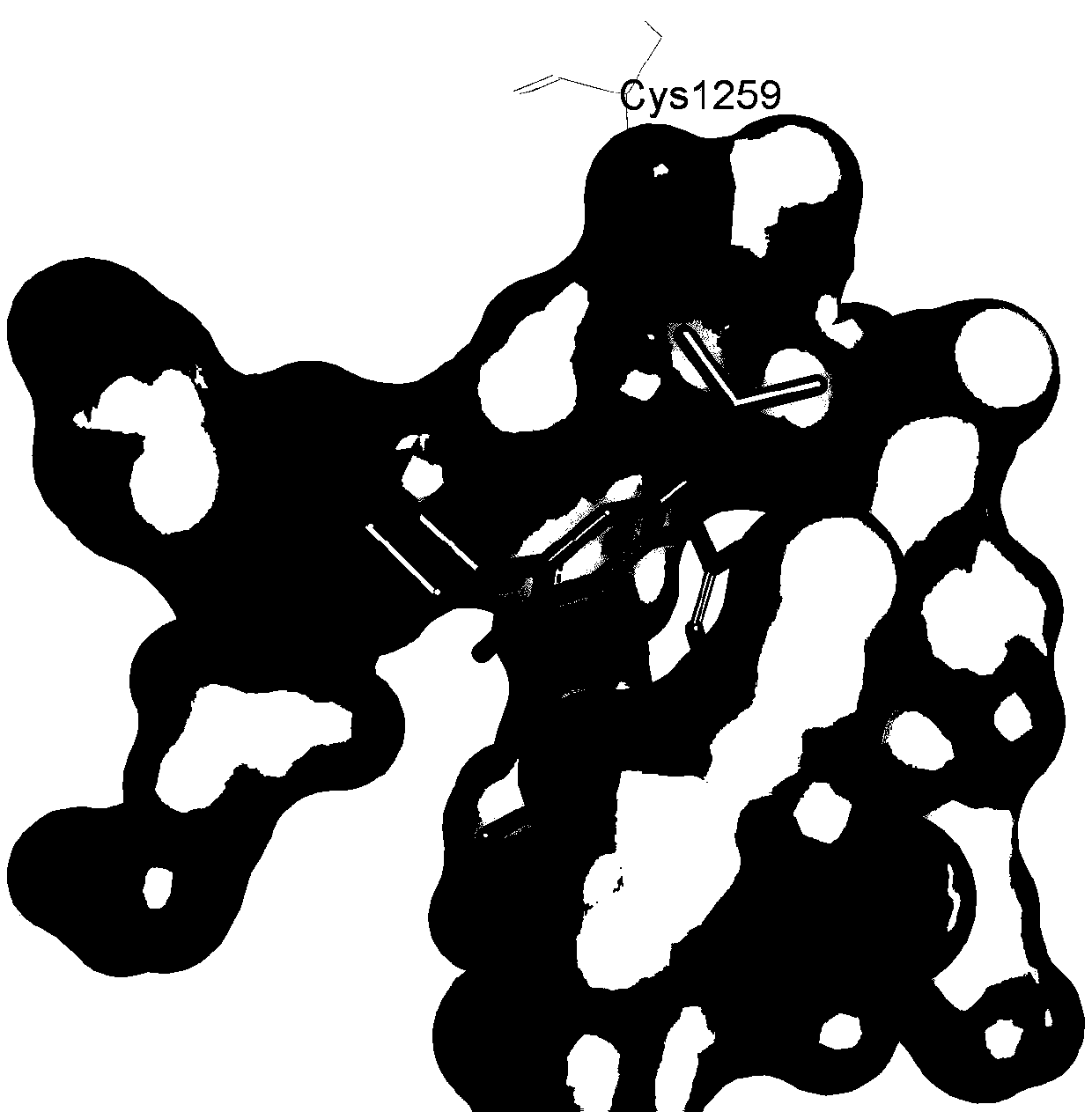
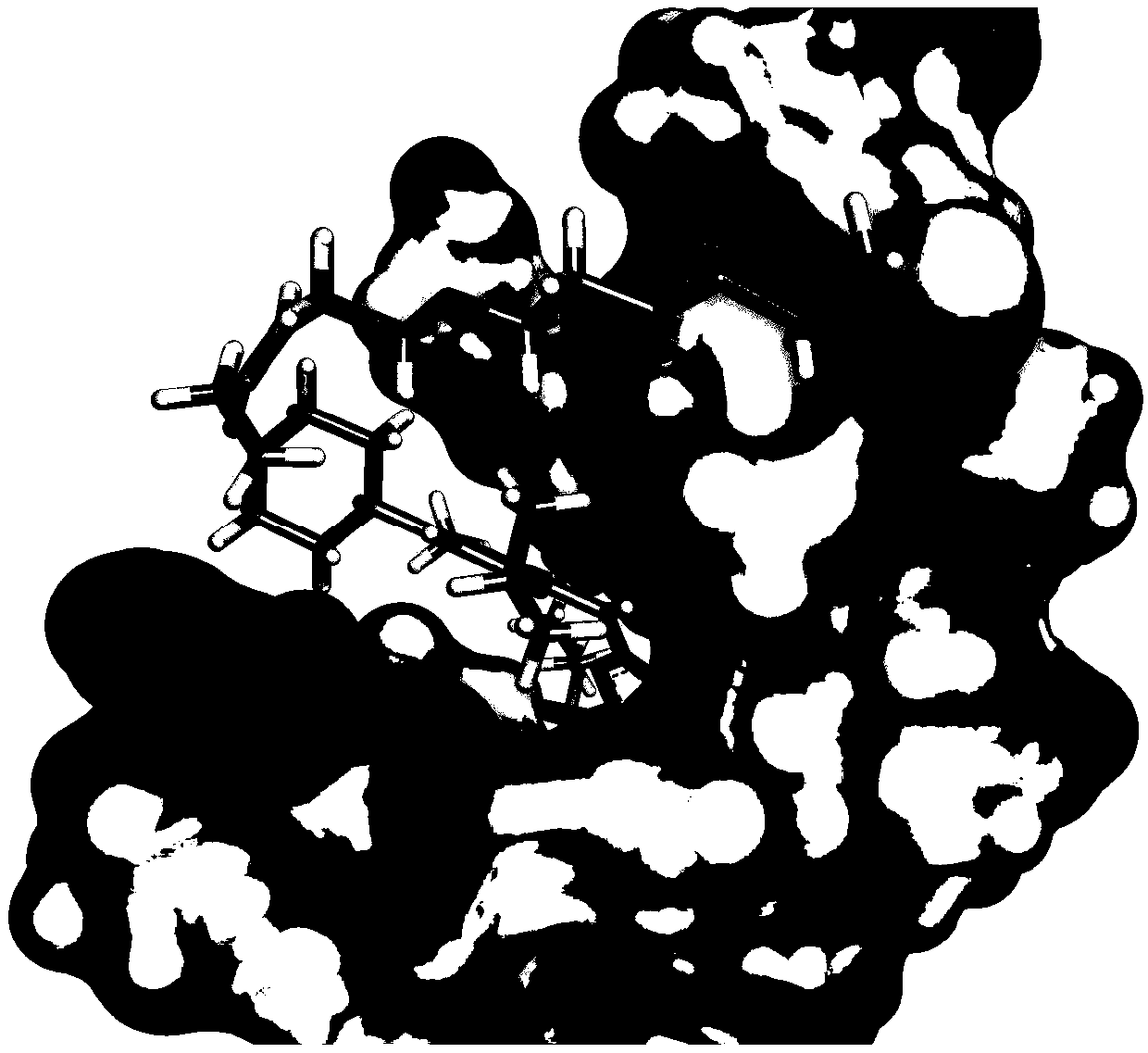
Design method of covalent drugs
A design method and drug technology, applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve problems such as high risk of side effects, obvious drug tolerance, and inability to design covalent drugs, achieve strong biological activity, improve The effect of binding capacity, increasing medication adherence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The synthesis of embodiment 1 compound ConA-1
[0048] 1. Synthesis of Intermediate 1
[0049]
[0050] Using LDK378 as raw material (330mg, 0.6mmol), dissolve it in 50mL of methanol, add methyl acrylate (78mg, 0.9mmol), triethylamine (240mg, 2.4mmol), stir at room temperature for 8h, and check the progress of the reaction by TLC. Treatment method: first remove methanol by distillation under reduced pressure, add 150mL ethyl acetate, backwash the organic solution three times with water, remove water with anhydrous sodium sulfate, concentrate under reduced pressure, and perform column chromatography with PE / EA separation system to obtain a white solid. The yield is about 90%.
[0051] 1 H NMR (400MHz, CDCl 3 )δ=9.49(s,1H),8.58(d,J=8.4,1H),8.15(s,1H),7.99(s,1H),7.93(d,J=8.0,1H),7.62(t, J=7.9,1H),7.53(s,1H),7.24(d,J=7.7,1H),6.80(s,1H),4.53(dt,J=12.1,6.0,1H),3.71(s,3H ),3.32–3.21(m,1H),3.06(d,J=9.6,2H),2.77(d,J=6.7,2H),2.72–2.52(m,3H),2.15(s,4H),1.76 (s,4H),1.34(dd...
Embodiment 2
[0064] The synthesis of embodiment 2 compound ConA-2
[0065] 1. Synthesis of intermediate ConA-2M
[0066]
[0067] The preparation method of this intermediate is the same as ConA-1M, the raw material is N-tert-butoxycarbonyl-1,4-butanediamine, and the other raw materials and treatment methods are the same. The yield was 76%.
[0068] 1 H NMR (400MHz, CDCl 3 )δ=6.27(dd, J=17.0,1.6,2H),6.13(dd,J=17.0,10.2,1H),5.62(dd,J=10.2,1.6,1H),4.72(s,1H),3.35 (q,J=6.4,2H),3.13(s,2H),1.62–1.49(m,4H),1.44(s,9H). 13 C NMR (101MHz, CDCl 3 )δ=165.74, 156.23, 130.99, 126.13, 79.27, 39.20, 28.41, 27.71, 26.50. HRMS (DART-TOF) calculated for C 12 h 23 N 2 o 3 [M+H] + m / z243.1709, found 243.1701.
[0069] 2. Synthesis of the target product ConA-2
[0070]
[0071] The preparation method of the target product is the same as ConA-1, the raw material is ConA-2M, and other raw materials and treatment methods are the same. The yield was 67%.
[0072] 1 H NMR (400MHz, CDCl 3 )δ=9.5...
Embodiment 3
[0073] The synthesis of embodiment 3 compound ConA-3
[0074] 1. Synthesis of intermediate ConA-3M
[0075]
[0076] The preparation method of this intermediate is the same as ConA-1M, the raw material is N-tert-butoxycarbonyl-1,6-hexanediamine, and the other raw materials and treatment methods are the same. The yield was 68%.
[0077] 1 H NMR (400MHz, CDCl 3 )δ=6.28(dd, J=17.0,1.4,1H),6.12(dd,J=17.0,10.2,1H),5.62(dd,J=10.2,1.3,1H),3.32(dd,J=13.1, 6.7,2H),3.11(dd,J=12.6,6.2,2H),1.60–1.41(m,13H),1.34(dd,J=8.8,5.6,4H).HRMS(DART-TOF) calculated for C 14 h 26 N 2 o 3 Na[M+Na] + m / z293.1841, found 293.1856.
[0078] 2. Synthesis of target product ConA-3
[0079]
[0080] The preparation method of the target product is the same as ConA-1, the raw material is ConA-3M, and other raw materials and treatment methods are the same. The yield was 67%.
[0081] 1 H NMR (400MHz, CDCl 3 )δ=9.52(s,1H),8.58(d,J=8.4,1H),8.16(s,1H),8.04(d,J=10.3,2H),7.93(dd,J=7.9,1.3,1H ),7.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com