Isosorbide mononitrate tablet and quality detection method thereof
A technology of isosorbide tablet and isosorbide dinitrate, which is applied in the field of medicine, can solve the problems that the slow-release effect needs to be improved, and achieve the effects of consistent stability, reasonable composition and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
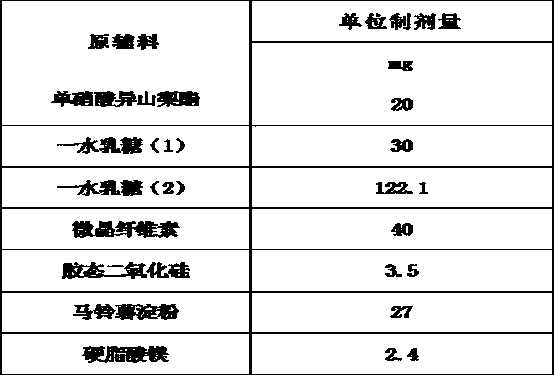
[0030] A kind of isosorbide mononitrate tablet, its formula is:
[0031]
[0032] The preparation process is as follows:
[0033] Pretreatment: If there is agglomeration of isosorbide mononitrate and colloidal silicon dioxide, use a 24-mesh sieve to remove the agglomerates. Single weighing: Weigh each raw and auxiliary material according to the prescription amount, and set aside.
[0034] Isosorbide dinitrate embedding: Add isosorbide mononitrate in the amount of 1g:10ml to carboxymethyl chitosan aqueous solution with a concentration of 30g / L, ultrasonically disperse for 10 min, and then add the same volume of Pan-80 liquid paraffin, stirred at 200 rpm for 10 min, cooled to 4°C, added 5% (v / v) glutaraldehyde to cross-link and solidify for 3 h, removed the oil layer, vacuum filtered, then washed with petroleum ether, and then Dehydrate with acetone and dry in a vacuum oven to obtain isosorbide mononitrate microspheres (drug loading 40%).
[0035] Pre-mixing: put the weigh...
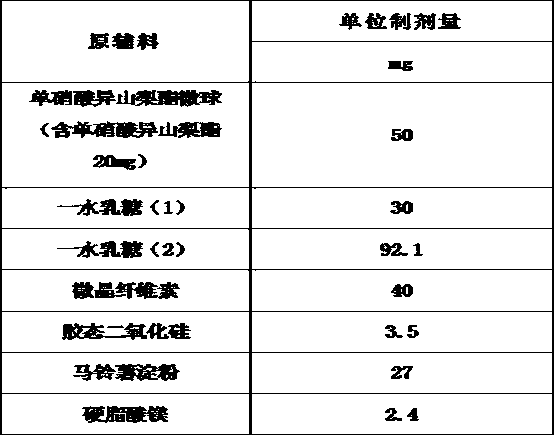
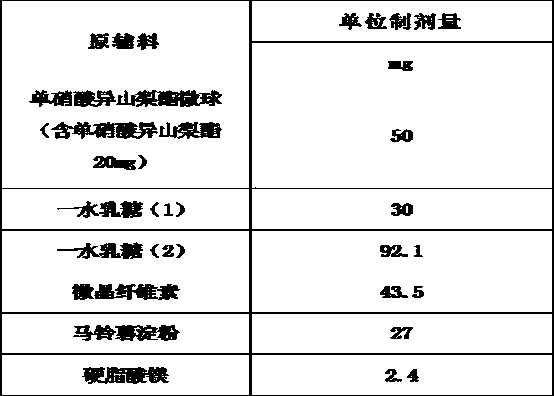
Embodiment 3
[0050] 1. Stability test:
[0051] Adopt the tablet among the embodiment 1 to carry out stability study, investigate the stability of placing 8 months under the condition of 80% relative humidity at 35 ℃ with the Isosorbide mononitrate tablet that this prescription process makes. They were measured at the 0th, 2nd, 4th, and 8th months respectively, see Table 1 for details.
[0052] Table 1
[0053] time month character content% Release% 0 white or off-white 100 qualified 2 white or off-white 100 qualified 4 white or off-white 99.9 qualified 8 white or off-white 99.7 qualified
[0054] Above stability test result shows, with the tablet of embodiment 1 through the accelerated test of 8 months, each index all reaches the standard, and its stability meets the requirements.
[0055] 2. In vitro release assay:
[0056] Chromatographic conditions: use octadecylsilane bonded silica gel as filler, water-methanol (25:75) as mobil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com