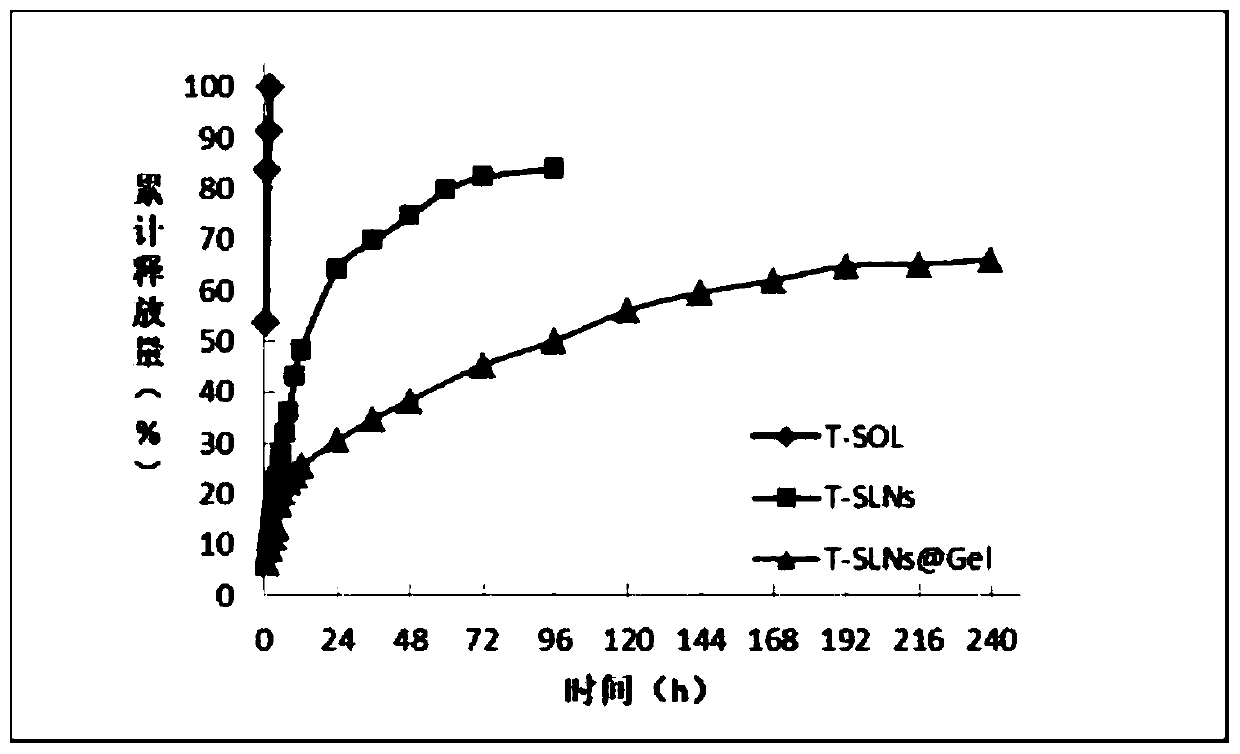
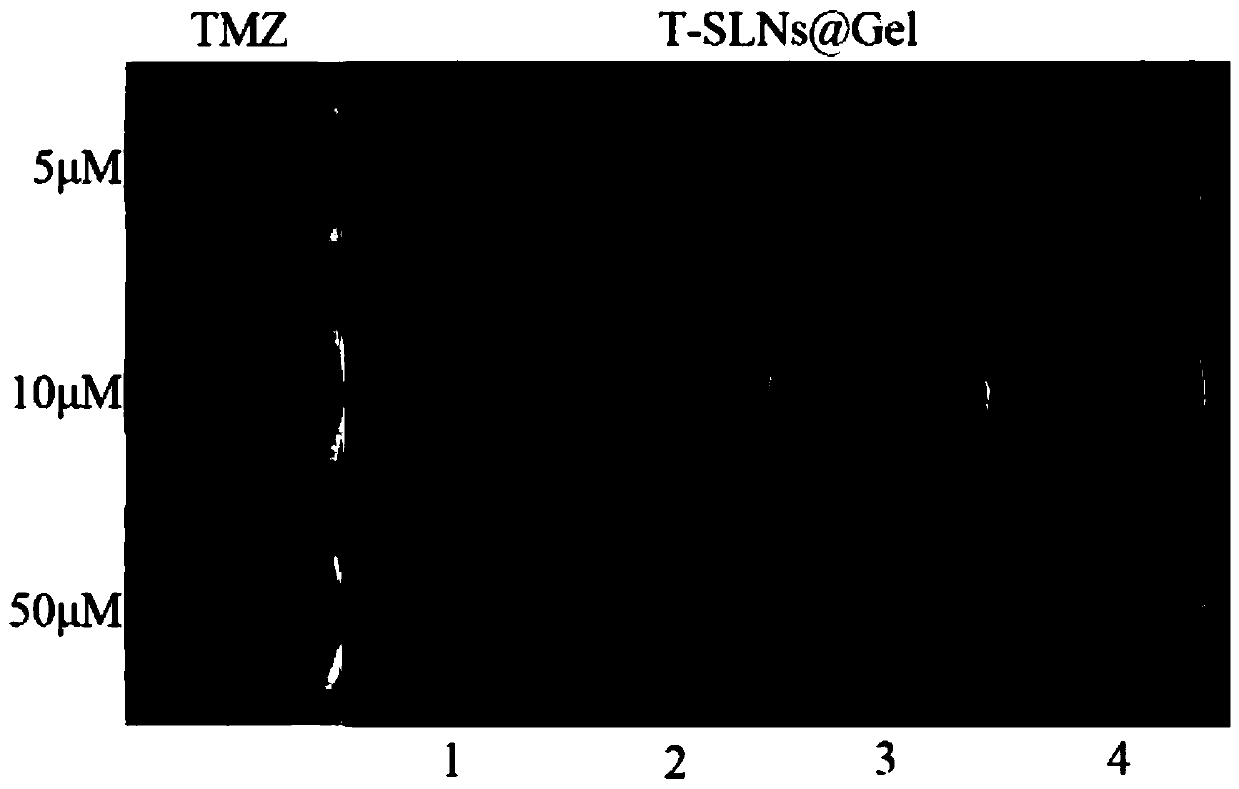
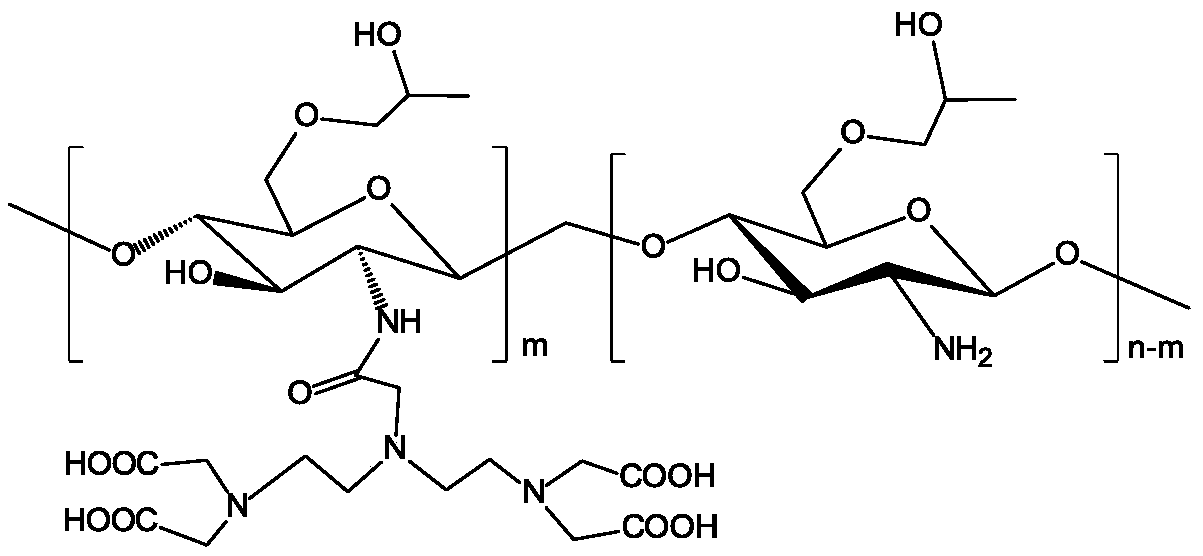
Temozolomide nuclear magnetic resonance visual injectable hydrogel as well as preparation method and application thereof
A temozolomide and nuclear magnetic resonance technology, applied in the field of temozolomide nuclear magnetic resonance visualized injectable hydrogel preparation, can solve the problem of less gel, avoid decomposition and leakage, reduce systemic side effects, and ensure drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Preparation of Temozolomide Solid Lipid Nanoparticles
[0046] (1) Under water-bath conditions, take by weighing 500 mg of solid lipid stearic acid and dissolve in an appropriate amount of chloroform under dark heat for subsequent use;
[0047] (2) Under water-bath conditions, weigh 100 mg of temozolomide and fully dissolve it in an appropriate amount of dimethyl sulfoxide (DMSO), and set aside;
[0048] (3) Add step (2) to step (1), mix well to form 5 mL of organic phase, set aside;
[0049] (4) Dissolve 250 mg of the emulsifier Poloxamer 188 in deionized water under water bath conditions to form 25 mL of the water phase (volume ratio of the organic phase to the water phase = 1:5), and set aside;
[0050] (5) Slowly add the organic phase prepared in step (3) dropwise to the aqueous phase obtained in step (4), stir rapidly (600r / min) during the dropwise addition to form the first emulsion, and set aside;
[0051] (6) Raise the temperature of the primary emulsion pr...
Embodiment 2
[0060] 1. Preparation of Temozolomide Solid Lipid Nanoparticles
[0061] (1) Under water bath conditions, weigh 250 mg of the solid lipid glyceryl behenate, dissolve it in an appropriate amount of chloroform and heat it away from light, and set aside;
[0062] (2) Under water-bath conditions, weigh 250 mg of temozolomide and fully dissolve it in an appropriate amount of dimethyl sulfoxide (DMSO) for subsequent use;
[0063] (3) Add step (2) to step (1), mix well to form 5 mL of organic phase, set aside;
[0064] (4) Dissolve 500 mg of Poloxamer 188 in deionized water under water bath conditions to form 25 mL of water phase (volume ratio of organic phase to water phase = 1:5) for later use;
[0065] (5) Slowly add the organic phase prepared in step (3) dropwise to the aqueous phase obtained in step (4), and stir rapidly (1200r / min) during the dropwise addition to form a primary emulsion for subsequent use;
[0066] (6) Raise the temperature of the primary emulsion prepared in...
Embodiment 3
[0072] 1. Preparation of Temozolomide Solid Lipid Nanoparticles
[0073] (1) Under water-bath conditions, weigh 650 mg of the solid lipid glyceryl monostearate, dissolve it in an appropriate amount of chloroform and heat it in the dark, and set aside;
[0074] (2) Under water-bath conditions, weigh 100 mg of temozolomide and fully dissolve it in an appropriate amount of dimethyl sulfoxide (DMSO), and set aside;
[0075] (3) Add step (2) to step (1), mix well to form 5 mL of organic phase, set aside;
[0076] (4) Dissolve 250 mg of the emulsifier Poloxamer 188 in deionized water under water bath conditions to form 25 mL of the water phase (volume ratio of the organic phase to the water phase = 1:5), and set aside;
[0077] (5) Slowly add the organic phase prepared in step (3) dropwise to the aqueous phase obtained in step (4), and stir rapidly (1200r / min) during the dropwise addition to form a primary emulsion for subsequent use;
[0078] (6) Raise the temperature of the prim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com