Large-sized ligustrazine phosphate drop pill
A Ligustrazine Phosphate, large-scale technology, applied in the field of large-scale Ligustrazine Phosphate dripping pills, can solve the problems of less drug loading, more pills to take, and incapable of industrial production, and achieve strong controllability of quality indicators and high technological efficiency. Good reproducibility and large drug loading effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
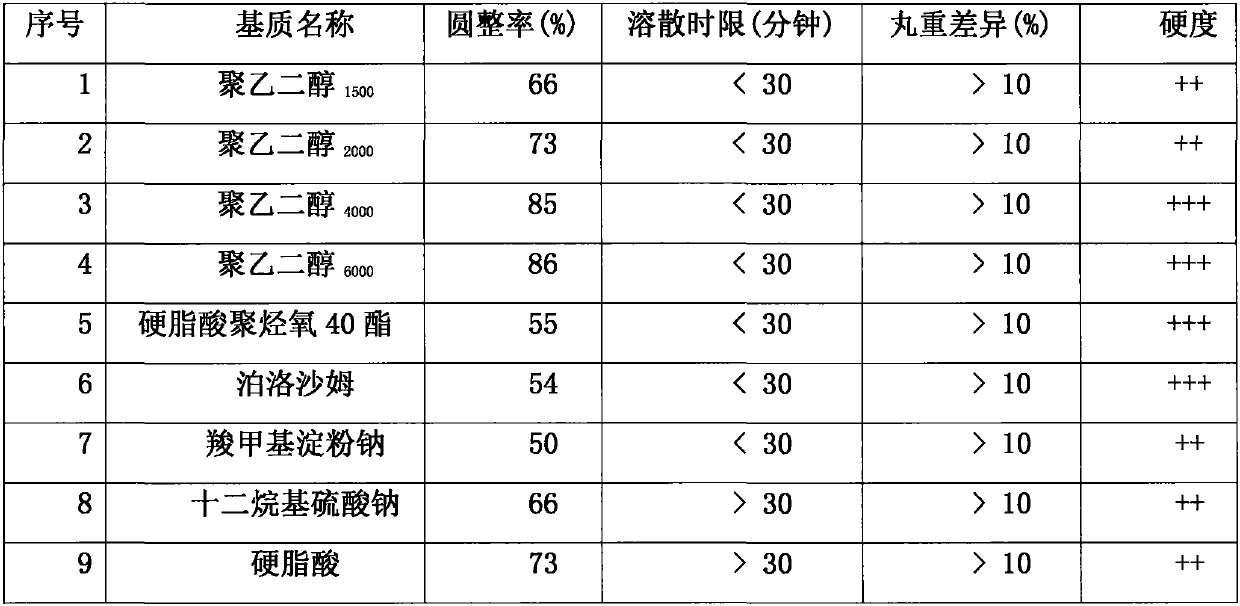
[0031] In order to investigate Ligustrazine Phosphate: the difference in quality of the drop pills made when the ratio of base is 1:1, according to the ratio of 1:1, Ligustrazine Phosphate was mixed with polyethylene glycol respectively. 1500 , polyethylene glycol 2000 , polyethylene glycol 4000 , polyethylene glycol 6000 , polyoxyl 40 stearate, poloxamer, sodium carboxymethyl starch, sodium lauryl sulfate, stearic acid, glycerin, gelatin and pharmaceutically acceptable carriers, according to the provisions in the "preparation method" According to the steps of preparation, 11 pharmaceutical composition experiments containing ligustrazine phosphate and different substrates can be obtained, and 11 groups of different experimental results are obtained, as shown in Table 1.
[0032] Table 1 Combination experiment of ligustrazine phosphate and single matrix
[0033] (Ligustrazine Phosphate: Matrix = 1:1)
[0034] serial number Matrix name Rounding rate (%) Dissol...
Embodiment 2
[0036] In order to investigate Ligustrazine Phosphate and the difference in quality of Ligustrazine Phosphate Dropping Pills prepared when the ratio of different substrates is 1: 3, according to the ratio of 1: 3, the ratio is carried out with the substrate described in Example 1, according to The preparation was carried out according to the steps specified in the "preparation method", and 11 groups of different experimental results were obtained, as shown in Table 2.
[0037] Table 2 Combination experiment of ligustrazine phosphate and single matrix
[0038] (Ligustrazine Phosphate: Matrix = 1:3)
[0039] serial number Matrix name Rounding rate (%) Dissolution time limit (minutes) Pill Weight Difference (%) hardness 1 polyethylene glycol 1500
Embodiment 3
[0041] In order to investigate Ligustrazine Phosphate and the difference in quality of Ligustrazine Phosphate Dropping Pills made when the ratio of different substrates is 1:5, according to the ratio of 1:5, the ratio is carried out with the substrate described in Example 1, according to The preparation was carried out according to the steps specified in the "preparation method", and 11 groups of different experimental results were obtained, as shown in Table 3.
[0042] Table 3 Combination experiment of ligustrazine phosphate and single matrix
[0043] (Ligustrazine Phosphate: Matrix = 1:5)
[0044]
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com