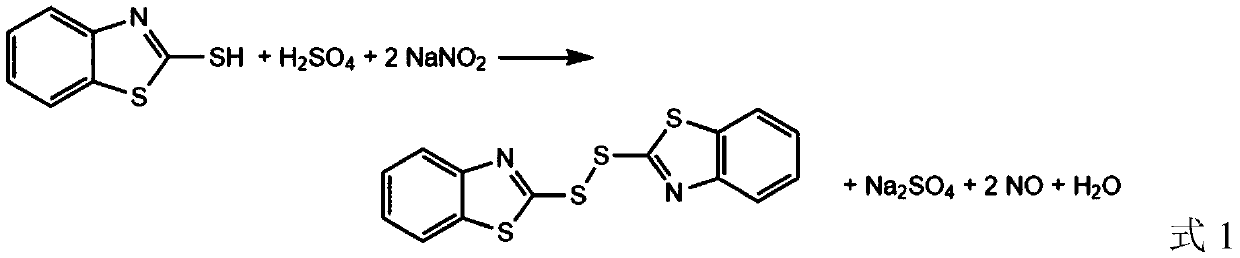
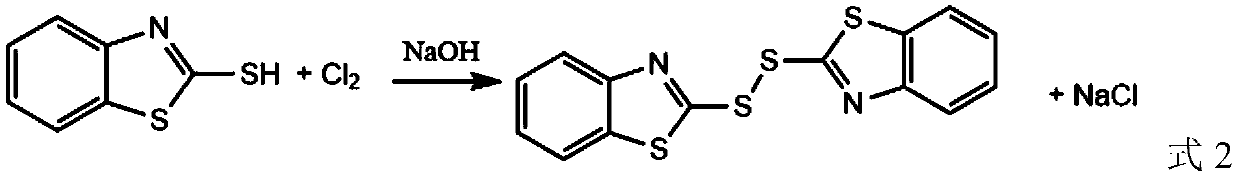
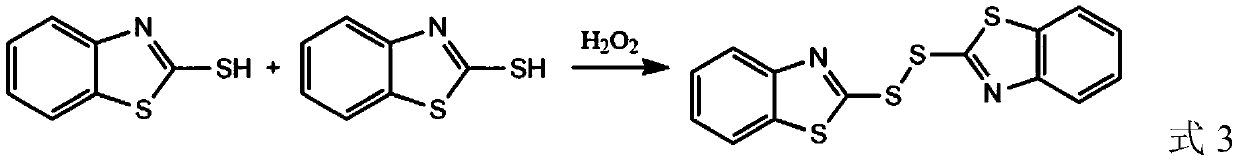
Green synthesis method of dibenzothiazole disulfide as rubber vulcanization accelerator
A technology of dibenzothiazole disulfide and rubber vulcanization, applied in reagents, organic chemistry, educts, etc., can solve the problems of poisonous and harmful reaction process, endanger human health, pollute air, etc., avoid peroxidation by-products, reduce Treatment costs and the effect of reducing the generation of saline wastewater
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Take 50 g of an aqueous sodium carbonate solution with a mass fraction of 15%, add 5.6 g of 2-mercaptobenzothiazole, and stir magnetically at a water bath temperature of 70° C. to completely dissolve the solid raw material to obtain a solution A containing 2-mercaptobenzothiazole sodium .
[0034] (2) Cool the solution A obtained in step (1) to 50°C, slowly add 37.07g of 1.37% H 2 o 2 Aqueous solution, and pass CO2 into the solution at a flow rate of 22.8mL / min 2 , after the reaction was carried out for 40min, it was cooled to room temperature to obtain a sodium bicarbonate solution B containing dibenzothiazole disulfide solid;
[0035] (3) The solution B obtained in the step (2) was suction filtered, and the obtained solid was washed with water and dried to obtain a solid product of dibenzothiazole disulfide, with a yield of 96.8% and a purity of 99.4% as detected by liquid chromatography.
Embodiment 2
[0037] (1) Take 50 g of an aqueous sodium carbonate solution with a mass fraction of 20%, add 5 g of 2-mercaptobenzothiazole, and stir magnetically at a water bath temperature of 80° C. to completely dissolve the solid material to obtain a solution A containing 2-mercaptobenzothiazole sodium;
[0038] (2) Cool the solution A obtained in step (1) to 40°C, and slowly add 33.89g of 1.5% H 2 o 2 Aqueous solution, and pass CO2 into the solution at a flow rate of 17.8mL / min 2 , after the reaction was carried out for 30min, it was cooled to room temperature to obtain a sodium bicarbonate solution B containing dibenzothiazole disulfide solid;
[0039] (3) The solution B obtained in step (2) was suction filtered, and the obtained solid was washed with water and dried to obtain a solid product of dibenzothiazole disulfide, with a yield of 97.8% and a purity of 99.1% as detected by liquid chromatography.
Embodiment 3
[0041] (1) Get the obtained filtrate of embodiment 2 step (3) i.e. sodium bicarbonate solution, obtain sodium carbonate solution after heating at 50°C, add 2-mercaptobenzothiazole 3g in the gained sodium carbonate solution, and magnetically Stir to completely dissolve the solid material to obtain a solution A containing 2-mercaptobenzothiazole sodium;
[0042] (2) Slowly add 22.37g of 1.50% H in solution A obtained in step (1) 2 o 2 Aqueous solution, and pass CO2 into the solution at a flow rate of 10.8mL / min 2 , the reaction was carried out after 60min and cooled to room temperature to obtain a sodium bicarbonate solution B containing dibenzothiazole disulfide solid;
[0043] (3) The solution B obtained in step (2) was suction filtered, and the obtained solid was washed with water and dried to obtain a solid product of dibenzothiazole disulfide with a yield of 97.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com