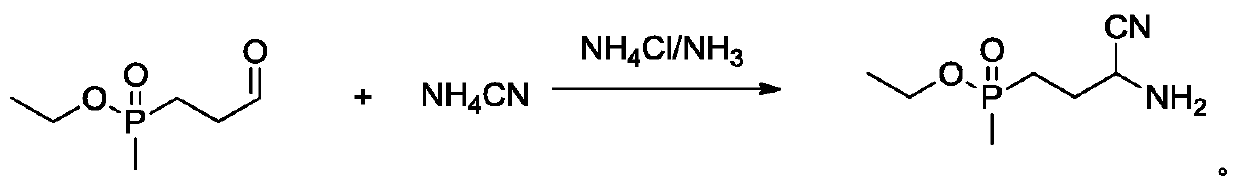
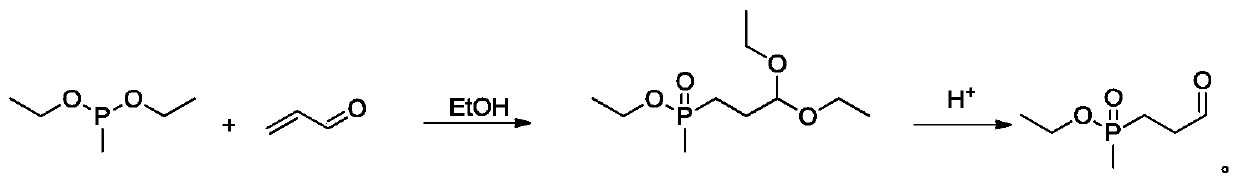
Synthesis method of glufosinate-ammonium intermediate
A synthesis method and intermediate technology, which are applied in the synthesis field of glufosinate-ammonium intermediates, can solve the problems of unsuitability for industrial production, high price, poor atom economy and the like, and achieve the advantages of avoiding the generation of mixed salts, easy operation and short reaction period. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthesis steps of ammonium cyanide aqueous solution are: dissolve 45g of sodium cyanide in 100g of water, add 73g of ammonium bicarbonate in batches, stir at 10-20°C for 1 hour after the addition, and filter out sodium bicarbonate to obtain ammonium cyanide Aqueous solution, wherein the mass content of ammonium cyanide is about 27%.
Embodiment 2
[0029] Add 150ml of ethanol and diethyl methylphosphite (68g, 0.5mol) in turn to the reaction flask, under the protection of nitrogen, add acrolein (28g, 0.5mol) dropwise at 15°C, and control the dropping time to 2h, After the dropwise addition, react at 25°C for 2 to 3 hours. After the reaction, distill under reduced pressure to obtain the residue 3-(methylethoxy)phosphoryl propionyl diethyl alcohol. Add 50g of 5% hydrochloric acid dropwise to the residue at 15°C, and control the dropping time for 2h. After the dropwise addition, keep the temperature at 20°C for 2h, and distill under reduced pressure after the reaction to obtain 78g of ethyl methylpropionyl phosphate , content 97.9%, yield 93.1%.
Embodiment 3
[0031] Add 27% ammonium cyanide aqueous solution (wherein containing ammonium cyanide 5.26g, 119.4mmol), ammonium chloride (6.45g, 119.4mmol) and 20% ammoniacal liquor 10g (wherein NH 3 119.4mmol), at 10°C, dropwise added ethyl methylpropionyl phosphate (10g, 59.7mmol) prepared in Example 2, after the dropwise addition, the temperature was raised to 30°C, reacted for 2 hours, and added 45g of 30% For industrial hydrochloric acid, the temperature was raised to reflux for 1 hour, the temperature was lowered to cool, ammonia was passed through, the ammonium chloride was filtered out, and methanol was added to crystallize to obtain 11.6 g of white glufosinate-ammonium aminonitrile solid with a content of 97% and a yield of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com