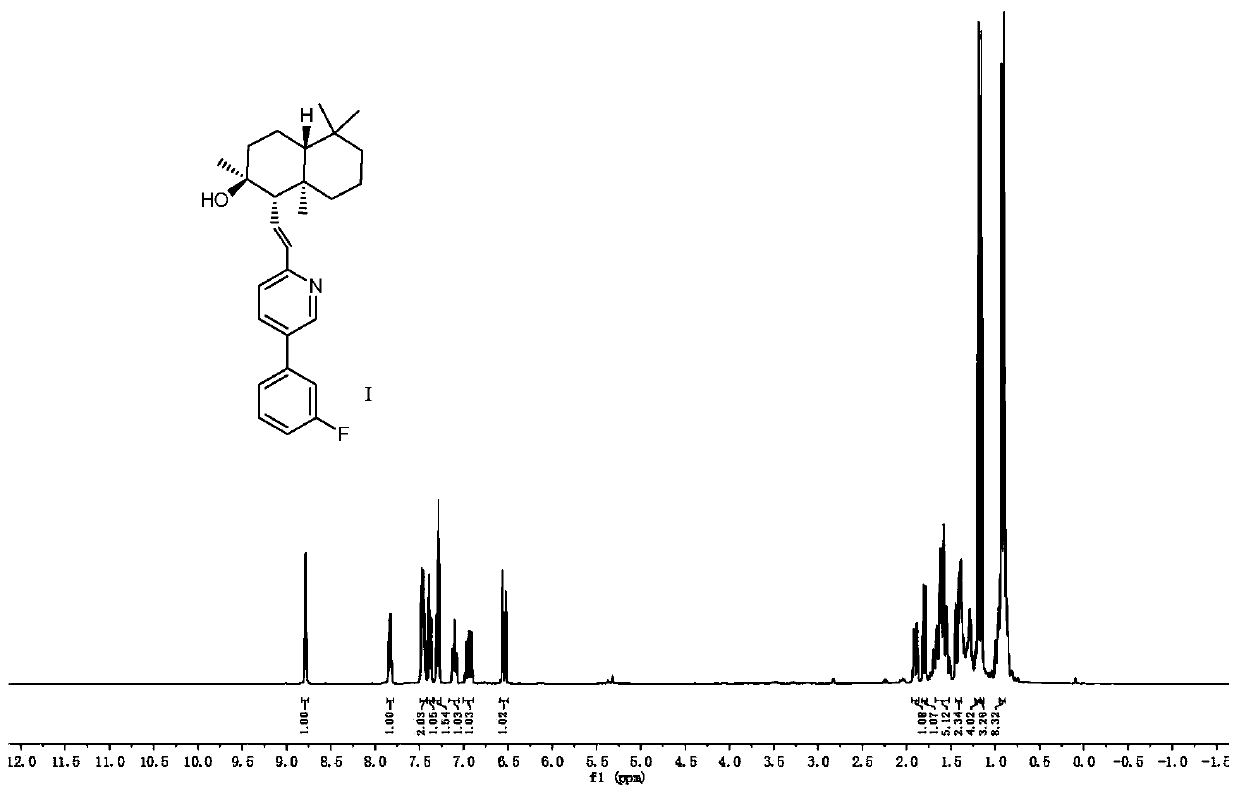
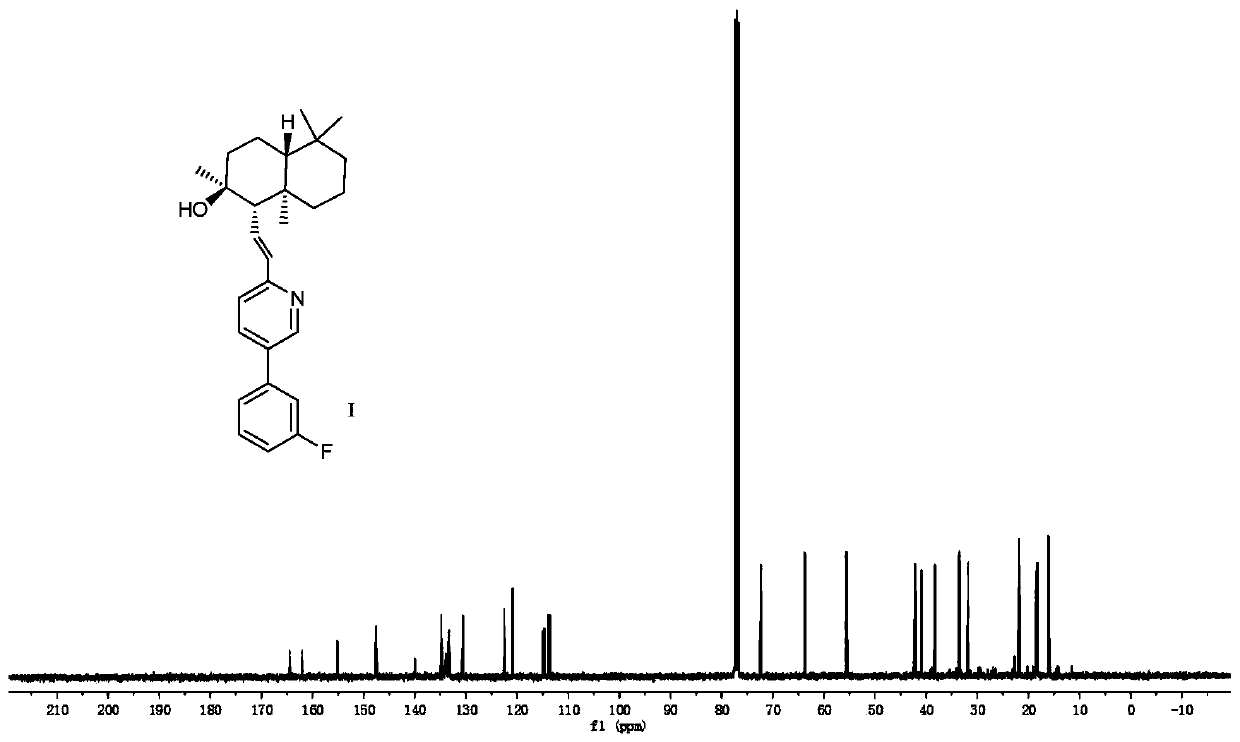
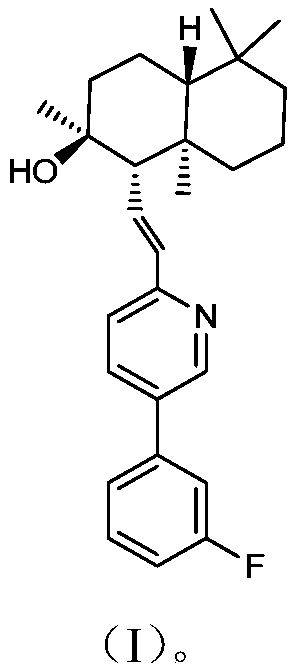
Novel PAR-1 inhibitor, preparation method and application thereof in prevention and/or treatment of thrombotic diseases
A technology for PAR-1 and thrombotic diseases, applied in the field of novel PAR-1 inhibitors and their preparation, can solve the problems of low activity of the compound and the value only reaches the micromolar level, etc., and achieves high activity, good economy, and easy industrialization. The effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation method of described novel PAR-1 inhibitor (compound I), concrete steps are as follows:
[0040] (1) Preparation of Compound IV
[0041]Weigh 4.20g of sclareolide and place it in a round bottom flask, add 75mL of tetrahydrofuran to dissolve it. Next, 25 mL of a 1M tetrahydrofuran solution of potassium hexamethyldisilazane and 2.8 mL of trimethoxyphosphine were slowly added to the round bottom flask in sequence. Finally, oxygen was introduced and reacted at -78°C. After the reaction was completed, the system was cooled down to room temperature, and the solvent was distilled off under reduced pressure to obtain compound II as a white solid. The prepared compound II was dissolved in 100 mL of anhydrous tetrahydrofuran, and placed in an ice-water bath to cool down to 2.5±2.5°C. Then weigh 1.60 g of lithium aluminum hydride and slowly add it into the round bottom flask. After the addition, the system was raised to room temperature and the reaction was comple...
Embodiment 2
[0045] The preparation method of described novel PAR-1 inhibitor (compound I), concrete steps are as follows:
[0046] (1) Preparation of Compound IV
[0047] Weigh 100.0 g of sclareolide and place it in a round bottom flask, add 1.7 L of tetrahydrofuran to dissolve it. Then, 595 mL of a 1M tetrahydrofuran solution of potassium hexamethyldisilazane and 67 mL of trimethoxyphosphine were slowly added to the round bottom flask in sequence. Finally, oxygen was introduced and reacted at -78°C. After the reaction was completed, the system was lowered to room temperature, and the solvent was evaporated under reduced pressure to obtain compound II as a white solid; after dissolving the prepared compound II in 24L anhydrous tetrahydrofuran, place Cool down to 2±1°C in an ice-water bath. Then weigh 38.1g of lithium aluminum hydride and slowly add it into the round bottom flask. After the addition, the system was raised to room temperature and the reaction was complete at room temperat...
Embodiment 3
[0051] Determination of biological activity (PAR-1 inhibitory activity) of compounds
[0052] 1. Cell culture
[0054] The HEK293-Gα15-PAR1 cell line (HD Biosciences stable cell line) was quickly taken out of the liquid nitrogen tank, and kept shaking in a 37°C water bath until it completely melted. Quickly add the cell suspension to the preheated medium (90% DMEM + 10% FBS + 1X Pen / Strep), put it into a centrifuge, and centrifuge at 1000 rpm for 10 minutes. Take out the centrifuge tube, discard the supernatant, add fresh preheated medium to the centrifuge tube, resuspend the cells, add the cell suspension to the culture dish, 37°C, 5% CO 2 nourish.
[0055] 1.2 Subculture
[0056] When the cells grow to 80-90% of the culture dish, gently wash the cells with 0.05% trypsin-EDTA, remove part of the digestion solution and incubate for 2-3 minutes, stop the digestion with a new medium, blow the cells gently with the tip of the pipette and Cells are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com