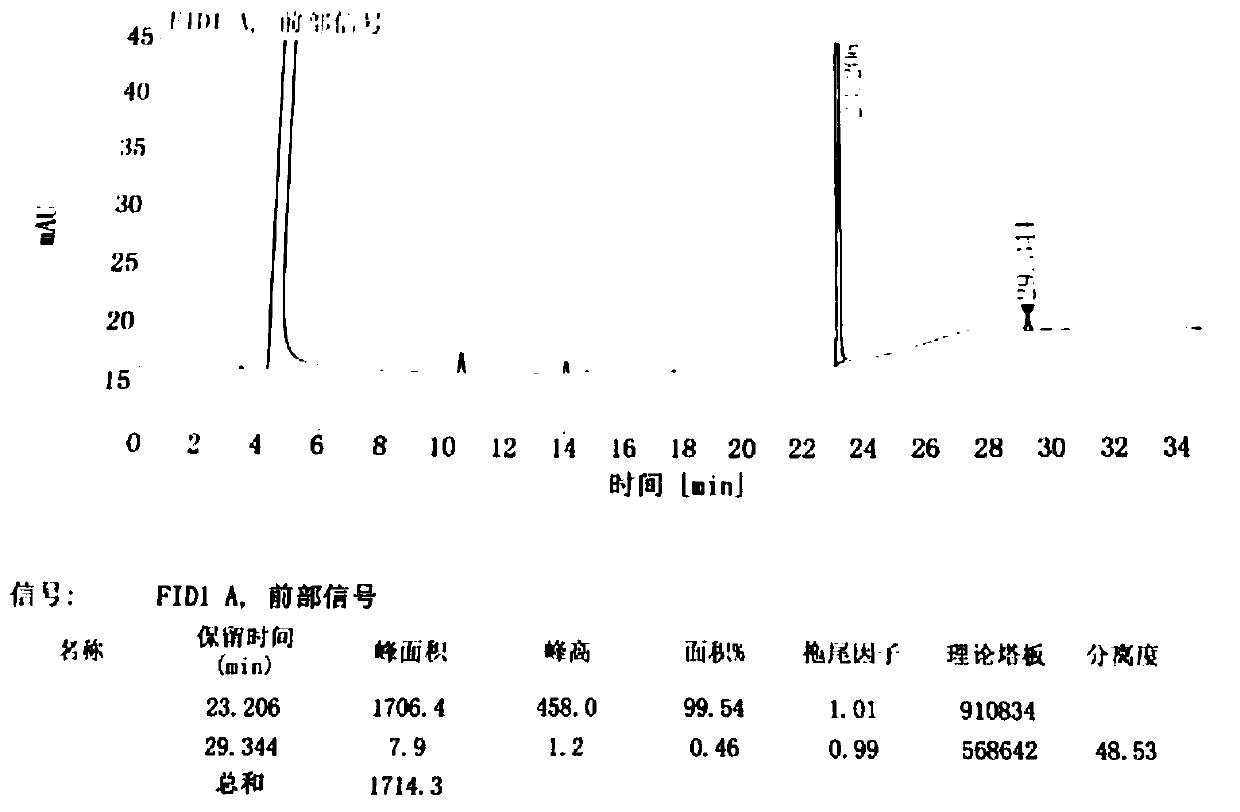
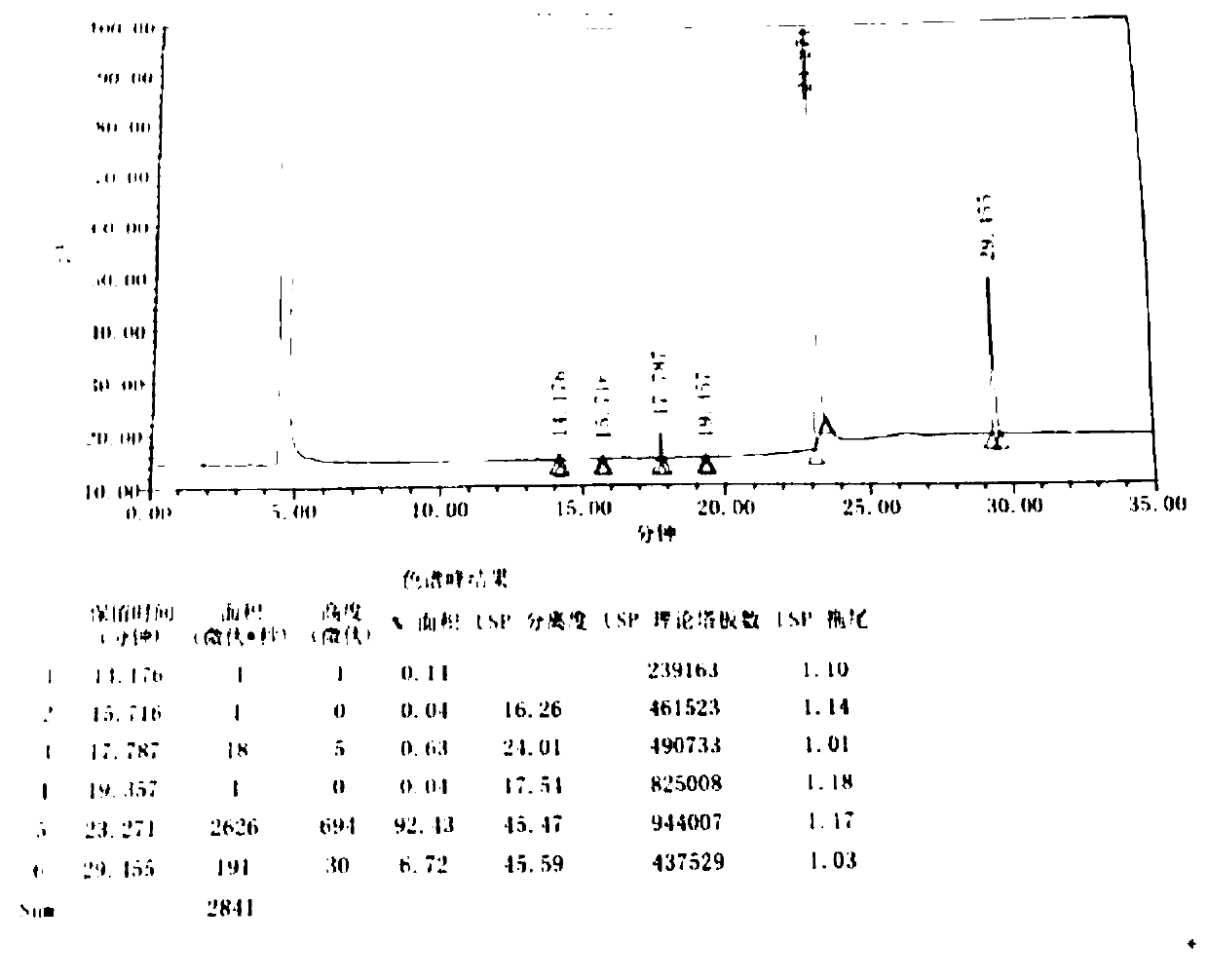
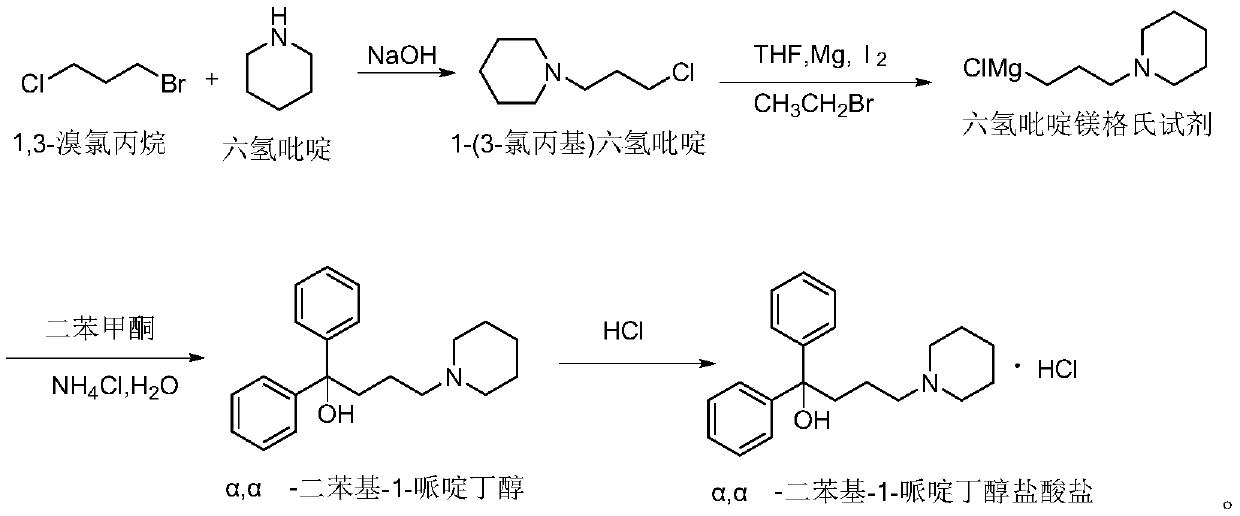
Preparation method of difenidol hydrochloride intermediate
A technology for diphenidol hydrochloride and intermediates, which is applied in the field of preparation of diphenidol hydrochloride intermediates, can solve the problems of long process steps, prolong drying time, consume a lot of time and the like, achieve less impurities and improve salt formation. rate, the effect of reducing the reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A preparation method of difenidol hydrochloride intermediate, that is, the intermediate is N-(3-chloropropyl) piperidine, and the specific process is:
[0039] S1. Put 160g of 1,3-bromochloropropane into the reaction tank, stir, and cool down to 14°C; slowly add 85g of hexahydropyridine dropwise to keep the reaction temperature in the temperature range of 30-45°C; add all the hexahydropyridine After the completion, the temperature was kept at 30°C for 2 hours; it took 3 hours;
[0040] S2. Slowly add 170g of lye (sodium hydroxide aqueous solution, concentration 30%) to the system, and keep warm at 30°C for 2 hours after adding; then cool down to below 17°C, slowly add about 30g of hydrochloric acid (about 12mol / L), and stir , so that pH = 2, maintain 15 ° C for 1 hour; it takes 4 hours;
[0041] S3. Centrifuge the reaction solution, return the solid product to other containers, add 0.8 times the volume (compared to the quality of the solid product) of ethyl acetate, an...
Embodiment 2
[0046] A preparation method of difenidol hydrochloride intermediate, that is, the intermediate is N-(3-chloropropyl) piperidine, and the specific process is:
[0047] S1. Put 160g of 1,3-bromochloropropane into the reaction tank, stir, and cool down to 15°C; slowly add 85g of hexahydropyridine dropwise to keep the reaction temperature in the temperature range of 30-45°C; add all the hexahydropyridine After the completion, the temperature was kept at 30°C for 2 hours; it took 3 hours;
[0048] S2. Slowly add 170g of lye (potassium hydroxide aqueous solution, concentration 30%) to the system, add and keep warm at 30°C for 2 hours; then cool down to below 17°C, slowly add 29g of hydrochloric acid (about 12mol / L), stir, Make pH = 3, maintain 15°C for 1 hour; it takes 4 hours;
[0049] S3. Centrifuge the reaction liquid to discharge the material, return the solid product to a specific container, add 1 times the volume (compared to the mass of the solid product) of acetone, and sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com