Preparation method of desloratadine medicine and desloratadine preparation
A technology of desloratadine and loratadine, which is applied in the field of medicine to achieve the effects of ensuring stability, improving stability, and solving process impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
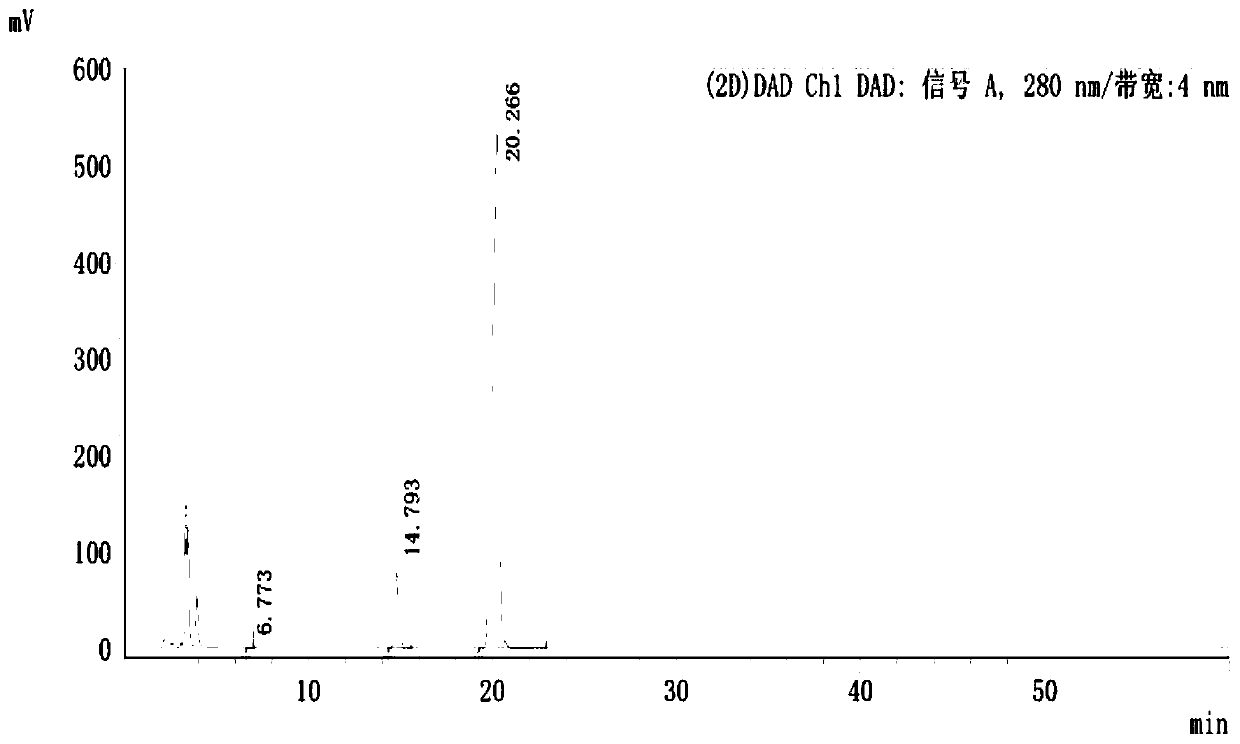
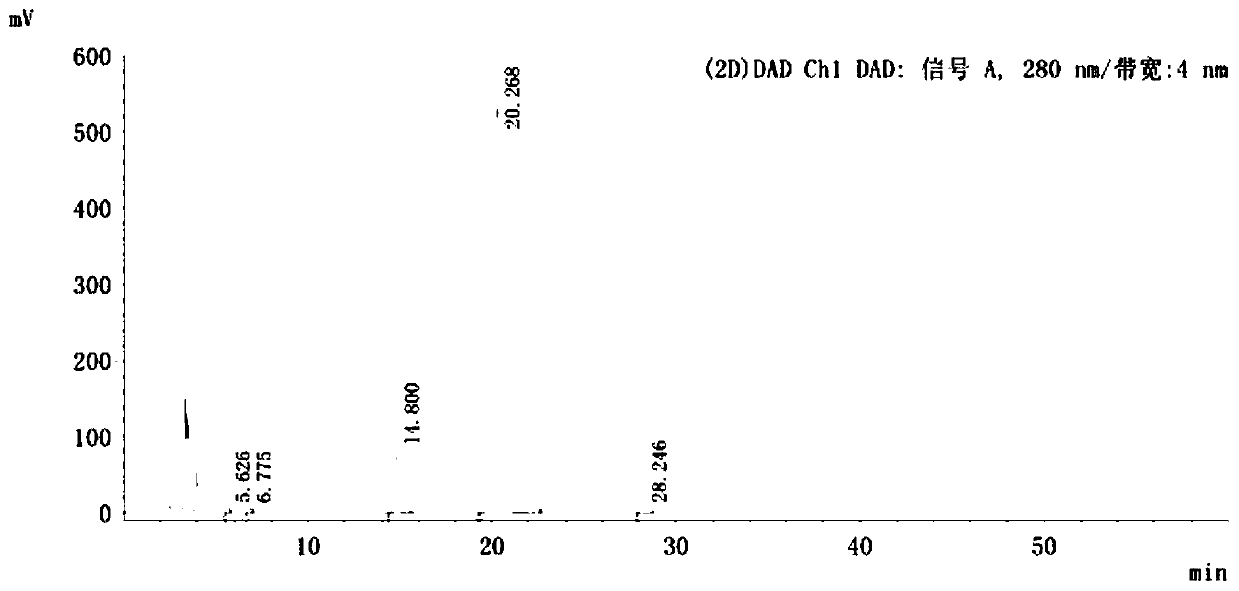
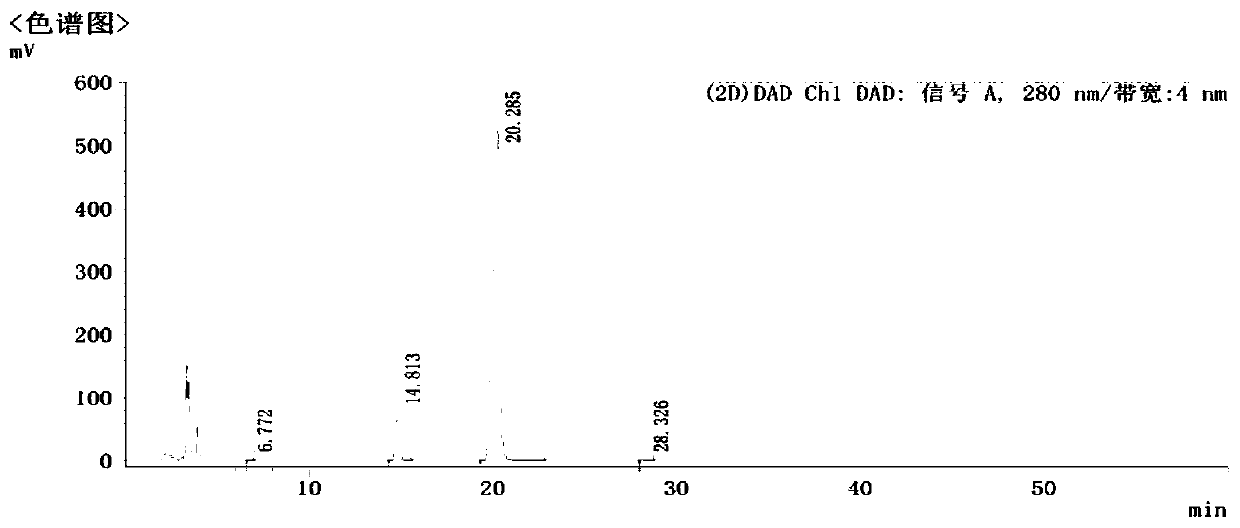
Image
Examples
preparation example Construction
[0043] The preparation method of desloratadine medicine of one embodiment, the method comprises the following steps:
[0044] S1: Add the organic acid salt to a co-solvent to dissolve, and then add the raw drug desloratadine for a mixed reaction;
[0045] The chemical name of desloratadine in this embodiment is 8-chloro-6,11-dihydro-11-(4-azicyclylidene)-5H-benzene-[5,6]-cyclohepta[1, 2-b]pyridine, molecular formula C 19 h 19 ClN 2 , the bulk drug can be a commercially available product, the structural formula is as follows:
[0046]
[0047] In one of the embodiments, S1 includes adding the organic acid salt into the organic co-solvent, dissolving, then adding the raw drug desloratadine, and diluting with water;
[0048]Specifically, add the organic acid salt into the organic co-solvent at room temperature, stir at a speed of 50r / min to 150r / min to completely dissolve, slowly add the raw drug desloratadine to completely dissolve, and add purified water to dilute ; The...
Embodiment 1
[0082] The preparation method of the desloratadine medicine of the present embodiment comprises the following steps:
[0083] 1) Add 100g of propylene glycol into the concentrated preparation tank at room temperature, add 1.2g of sodium citrate, stir at a speed of 100r / min to completely dissolve, slowly add 0.5g of the raw material desloratadine to completely dissolve, and add Dilute with 200mL purified water;
[0084] 2) Add 500mL of purified water to the main liquid mixing tank at room temperature, stir at a speed of 100r / min, and add 7g of hypromellose in batches. After it is completely dissolved, add 150g of sorbitol and 0.45g of citric acid 1. Edetate disodium 0.25g, stirred until completely dissolved;
[0085] 3) Transfer the solution in the medium concentration mixing tank of step (1) to the main liquid mixing tank of step (2), stir at room temperature for 5 minutes at a speed of 100 r / min, add 2 g of sucralose and 5 g of strawberry flavor essence , after stirring for...
Embodiment 2
[0091] The preparation method of the desloratadine medicine of the present embodiment comprises the following steps:
[0092] 1) Add 100g of polyethylene glycol into the concentrated preparation tank at room temperature, add 1.2g of organic carboxylate sodium citrate, stir at a speed of 100r / min to completely dissolve, and slowly add the raw drug desloratadine 0.5g to dissolve completely, and add 200mL purified water to dilute;
[0093] 2) Add 500mL of purified water to the main liquid mixing tank at room temperature, stir at a speed of 100r / min, and add 7g of hydroxyethyl cellulose in batches. After it is completely dissolved, add 150g of xylitol and 0.45g of citric acid g. 0.25 g of disodium edetate, an ion regulator, was stirred until completely dissolved.
[0094] 3) Transfer the solution in the medium-concentration mixing tank of step 1) to the main mixing tank of step 2). After stirring for 5 minutes at a speed of 100 r / min at room temperature, add 2 g of sweetener sucr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com