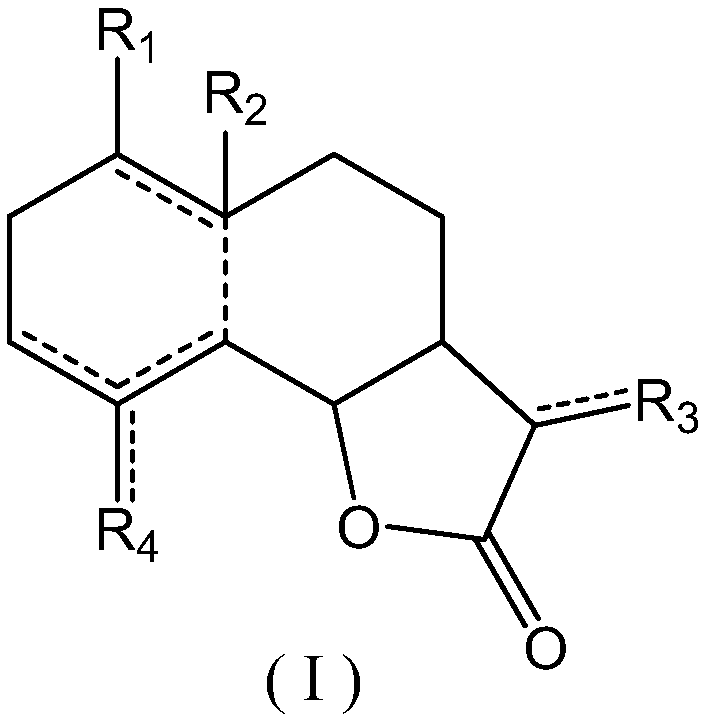
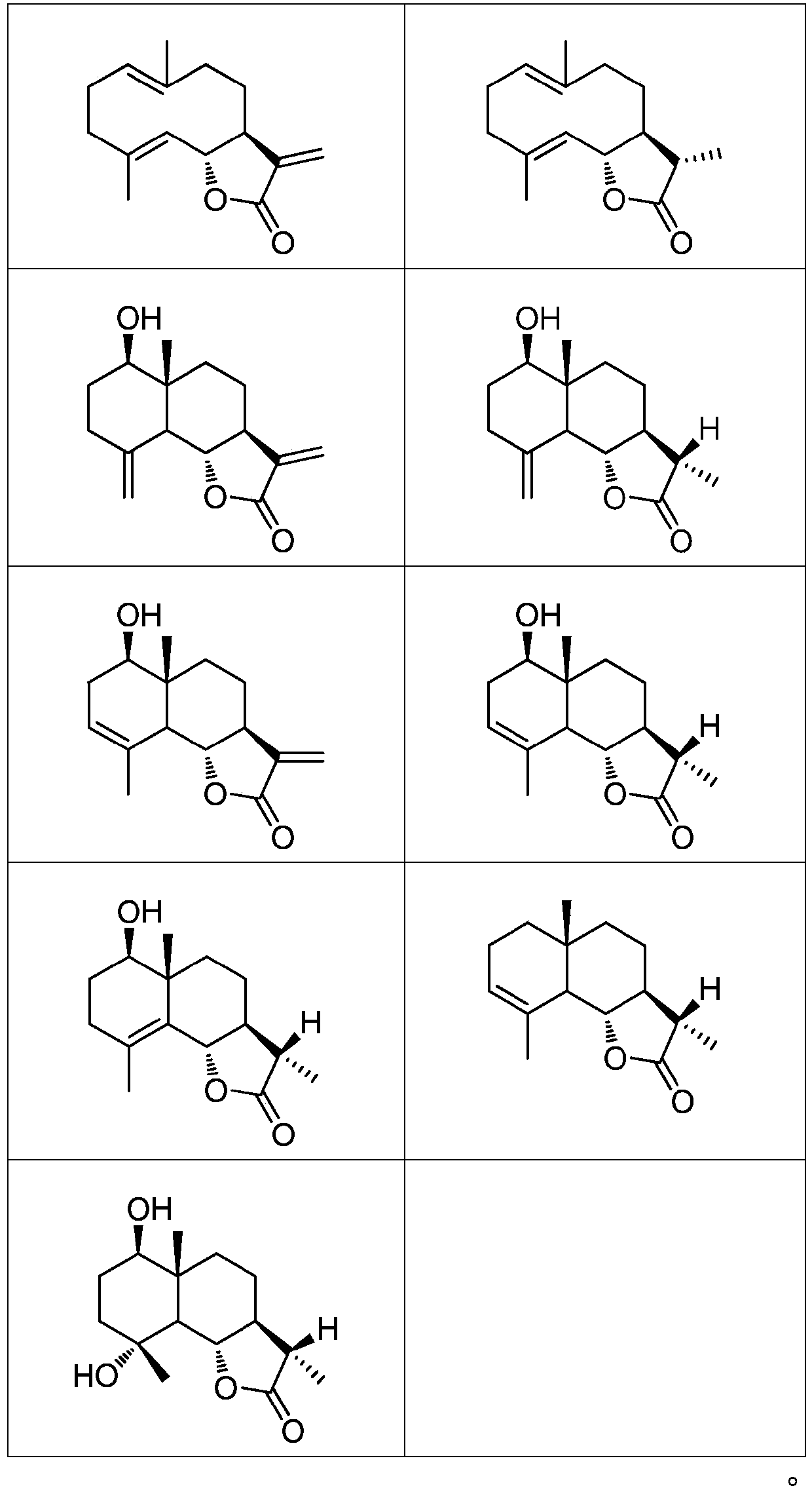
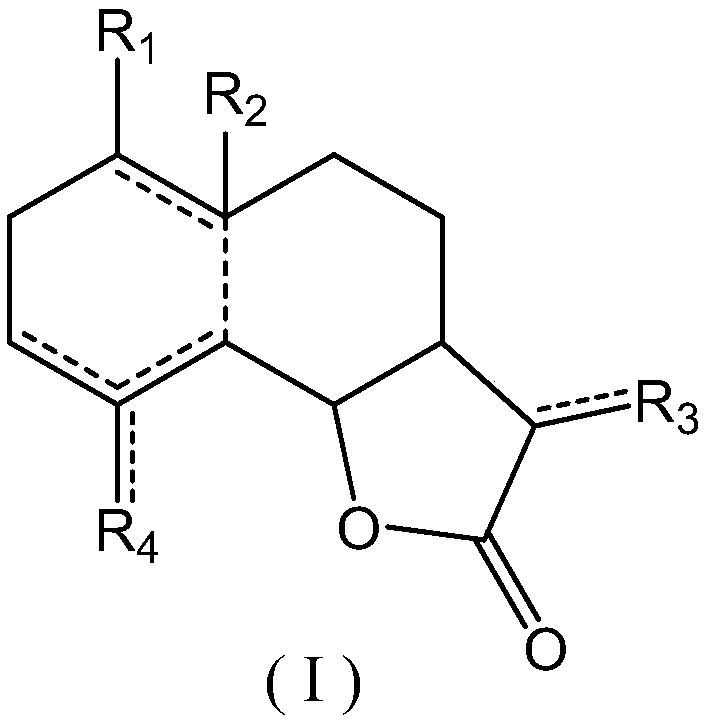
New application of sesquiterpenoids to uric acid lowering
A technology of sesquiterpenoids and compounds is applied in the field of new uses of sesquiterpenoids in lowering uric acid, which can solve the problems of triggering acute gout attacks, low drug tolerance, and increasing the risk of cardiovascular system diseases.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Weigh 50 kg of inula chinensis medicinal material, pulverize the inulin inulin, soak and extract 3 times with 8 times the volume of ethanol solution with a volume fraction of 90%, and concentrate under reduced pressure to remove the organic solvent to obtain concentrated solution A; pass the concentrated solution A over 50 L -D101 column (column diameter 22cm × height 150cm), wash 4 column volumes respectively with volume fraction of 40% and 95% ethanol, collect the eluate with volume fraction of 95% ethanol, and concentrate under reduced pressure to remove the organic solvent to obtain Concentrated solution B (solid content in concentrated solution B is 3.2kg), concentrated solution B was extracted with petroleum ether for 3 times, and the extraction solvent was recovered under reduced pressure to obtain 2 kg of petroleum ether extract. The petroleum ether eluate was separated by silica gel column chromatography, and gradient elution was carried out with a mixed solutio...
experiment example 1
[0052] Experimental example 1 Research on the Uric Acid-lowering Effect of the Compounds of the Invention
[0053] 1. Experimental materials
[0054] 150 healthy male KM mice, weighing 15-18 g, were provided by Beijing Weitong Lihua Biotechnology Co., Ltd.; 5 mice per cage were divided into cages, and then adaptively reared in the barrier system for 4 days.
[0055] 2. Experimental method
[0056] 2.1 Experimental grouping
[0057] Select 120 mice with concentrated body weight from 150 mice and randomly divide them into 12 groups according to body weight, with 10 mice in each group, which are blank control group, model control group, positive control group, and experimental groups 1-9.
[0058] 2.2 Administration method
[0059] Immediately after the adaptation period, the mice were intragastrically administered once a day in the morning for 7 consecutive days.
[0060] Experimental groups 1-9 were given 25 mg / kg of compound 1-9, and compound 1-9 were suspended with 0.5%...
experiment example 2
[0079] Experimental example 2 Research on the effect of the compound of the present invention on promoting uric acid excretion
[0080] 1. Experimental materials
[0081] 150 healthy male KM mice, weighing 15-18 g, were provided by Beijing Weitong Lihua Biotechnology Co., Ltd.; 5 mice per cage were divided into cages, and then adaptively reared in the barrier system for 4 days.
[0082] 2. Experimental method
[0083] 2.1 Experimental grouping
[0084] Select 120 mice with concentrated body weight from 150 mice and randomly divide them into 12 groups according to body weight, with 10 mice in each group, which are blank control group, model control group, positive control group, and experimental groups 1-9.
[0085] 2.2 Administration method
[0086] Immediately after the adaptation period, the mice were intragastrically administered once a day in the morning for 6 consecutive days.
[0087]Experimental group 1-9 groups were given compound 1-9 25mg / kg respectively, suspen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com