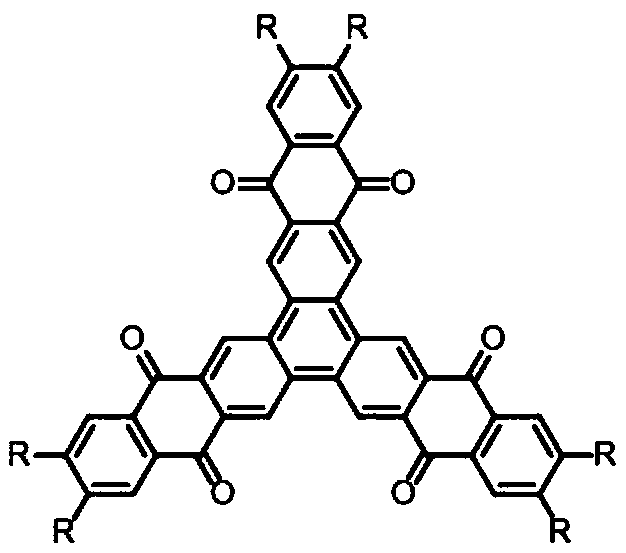
N-type star-shaped polycyclic conjugated aromatic hydrocarbon and synthesis method thereof
A synthesis method, N-type star technology, applied in the field of organic liquid crystal materials, can solve the problems of scarcity, etc., and achieve the effect of simple operation, simple and easy-to-obtain raw materials, and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
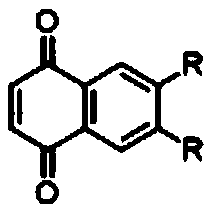
[0041] Compound A is 5,6-di-n-hexyl-1,4-naphthoquinone, R is -C 6 h 13 .
[0042] A kind of N-type star polycyclic conjugated aromatic hydrocarbon, its structural formula is as follows:
[0043]
[0044] The synthesis method of the above-mentioned N-type star-shaped polycyclic conjugated aromatic hydrocarbons specifically comprises the following steps:
[0045] (1) Synthesis of hexabromomethylbenzene
[0046] Add 1.62g of hexamethylbenzene and 30mL of 1,2-dibromoethane to a 100mL single-necked bottle in turn, raise the temperature to 130°C, stir until the hexamethylbenzene is completely dissolved, and slowly add 3.25mL of liquid bromine (Br 2 ) and 10mL of 1,2-dibromoethane, after the dropwise addition, maintain reflux reaction at 130°C for 30h. Then cool down to room temperature naturally, add dropwise 30mL 0.50mol / LNaHSO 3 The solution quenched the reaction to remove excess liquid bromine, filtered, and then washed three times with deionized water and acetone until t...
Embodiment 2
[0050] Compound A is 5,6-bis(4-tert-butylphenyl)-1,4-naphthoquinone, R is
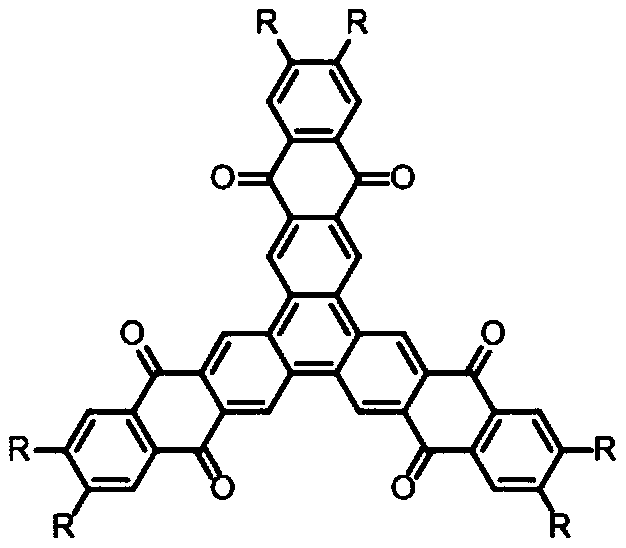
[0051] A kind of N-type star polycyclic conjugated aromatic hydrocarbon, its structural formula is as follows:
[0052]
[0053] Using the hexabromomethylbenzene synthesized in Example 1 to synthesize the above-mentioned N-type star-shaped polycyclic conjugated aromatic hydrocarbons.
[0054] 100mL three-necked flask vacuum-gas three cycles, add 2.11g 5,6-di(4-tert-butylphenyl)-1,4-naphthoquinone, 0.95g hexabromomethylbenzene, 3.37g iodide Sodium (NaI) and 45mL N,N-dimethylformamide (DMF) were heated to 90°C and reacted for 48h under nitrogen protection. Cool to room temperature, add 40 mL of acetone, stir for 30 min, filter, wash with deionized water and acetone until the filtrate is colorless, collect the filter cake, dry and column chromatography to obtain 0.91 g of a yellow solid, which is the above-mentioned N-type star Polycyclic conjugated aromatic hydrocarbons, yield 43%.
Embodiment 3
[0056]Compound A is 5,6-di(n-hexylcarboxylate)-1,4-naphthoquinone, R is -COOC 6 h 13 .
[0057] A kind of N-type star polycyclic conjugated aromatic hydrocarbon, its structural formula is as follows:
[0058]
[0059] Using the hexabromomethylbenzene synthesized in Example 1 to synthesize the above-mentioned N-type star-shaped polycyclic conjugated aromatic hydrocarbons.
[0060] 100mL there-necked flask was vacuumed-nitrogen for three cycles, and then 2.07g of 5,6-bis(n-hexylcarboxylate)-1,4-naphthoquinone, 0.95g of hexabromomethylbenzene, 3.37g of sodium iodide ( NaI) and 45mL N,N-dimethylformamide (DMF), heated to 90°C, and reacted for 48h under nitrogen protection. Cool to room temperature, add 40 mL of acetone, stir for 30 min, filter, wash with deionized water and acetone until the filtrate is colorless, collect the filter cake, dry and column chromatography to obtain 1.08 g of yellow solid, which is the above-mentioned N-type star Polycyclic conjugated aromatic h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com