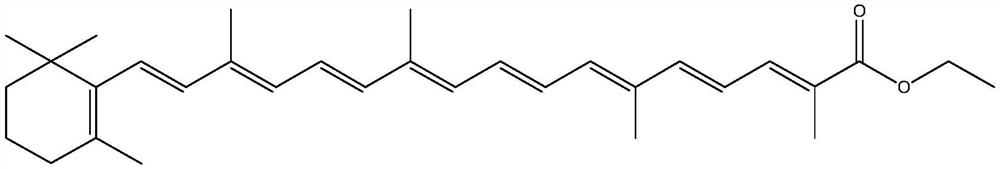
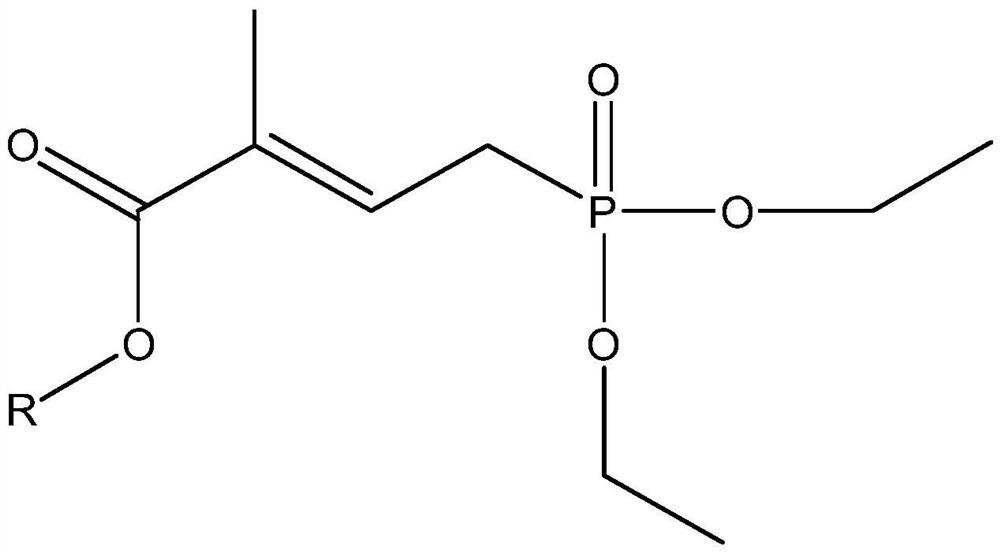
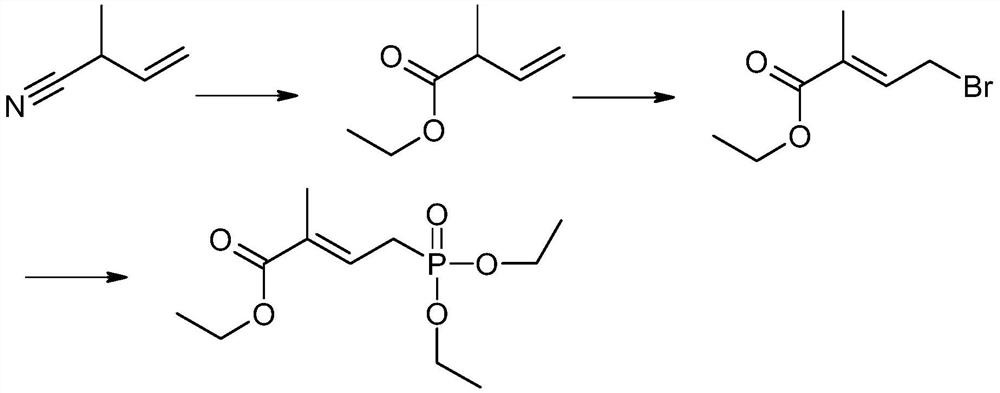
The preparation method of 4-(diethoxy-phosphoryl)-2-methyl-but-2-enoate
A technology of diethoxy and phosphoryl, which is applied in the field of preparation of organic synthesis intermediates, can solve the problems of low total yield, strong corrosive waste gas discharge, strong oxidative waste water discharge, etc., and achieves huge environmental protection benefits and simple separation. , the effect of saving organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] In reaction vessel, add 40% (wt%) the aqueous solution of aceguvaldehyde aqueous solution 162g (0.90mol) and 49 grams of sodium carbonate solid configurations, add palladium bismuth molybdenum / carbon catalyst 3.6g. Oxygen was introduced into the reaction solution, the temperature was raised to 40° C., the reaction was stirred for 3 hours, the stirring was stopped and filtered to obtain an aqueous solution of pyruvic acid / sodium pyruvic acid.
[0053] Dissolve 50 grams of sodium ethoxide in 300 grams of ethanol, then slowly add dropwise to 218.7 g (0.72 mol) of tetraethyl ethylene diphosphate dissolved in 150 ml of dichloromethane solution, stir for 10 minutes after the addition is complete, then drop rapidly Add pyruvic acid / sodium pyruvic acid aqueous solution, keep warm in a water bath at room temperature (20°C, within plus or minus 2°C), react for 30 minutes, and then pass in carbon dioxide for acidification. After the acidification, the liquid was separated, and the...
Embodiment 2
[0056] Add the aqueous solution of 40% (wt%) aceglyoxal aqueous solution 162g (0.9mol) and 80 gram solid sodium bicarbonate configurations in reaction vessel, add palladium bismuth molybdenum / carbon catalyst 3.6g. Oxygen was introduced into the reaction solution, and the temperature was raised to 20°C. The reaction time is 6h. Stop stirring and filter to obtain pyruvic acid / sodium pyruvic acid aqueous solution.
[0057] Dissolve 50 grams of sodium ethoxide in 300 grams of ethanol, then slowly add dropwise to 218.7 g (0.72 mol) of tetraethyl ethylene diphosphate dissolved in 150 ml of dichloromethane solution, stir for 10 minutes after the addition is complete, then drop rapidly Add pyruvic acid / sodium pyruvic acid aqueous solution, keep warm at 0°C with a freezing tank, react for 60 minutes, and then pass in carbon dioxide for acidification. After the acidification, the liquid was separated, and the oil layer was taken for the next reaction.
[0058] 140 ml of ethanol was a...
Embodiment 3
[0060] Add the aqueous solution of 40% (wt%) aceglyoxal aqueous solution 162g (0.9mol) and 37 sodium hydroxide configurations in reaction vessel, add palladium bismuth molybdenum / carbon catalyst 3.6g. Oxygen was introduced into the reaction liquid, and the temperature was raised to 60°C. Reaction time 3h. Stop stirring and filter to obtain pyruvic acid / sodium pyruvic acid aqueous solution.
[0061] Dissolve 50 grams of sodium ethoxide in 300 grams of ethanol, then slowly add dropwise to 218.7 g (0.72 mol) of tetraethyl ethylene diphosphate dissolved in 150 ml of dichloromethane solution, stir for 10 minutes after the addition is complete, then drop rapidly Add pyruvic acid / sodium pyruvate aqueous solution, keep warm in a water bath at room temperature (30°C, within plus or minus 2°C), react for 20 minutes, and then pass in carbon dioxide for acidification. After the acidification, the liquid was separated, and the oil layer was taken for the next reaction.
[0062] 80 ml of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com