Antibody preparation for preventing and treating bovine viral diarrhea and preparation method thereof
A bovine viral diarrhea and antibody technology is applied in the direction of antiviral agents, antibodies, and medical preparations with non-active ingredients, etc. It can solve the problems of low utilization rate of drugs, structural denaturation of antibodies, and discounted effects, so as to facilitate the supply of drinking water. medicine, accelerated recovery, and safe use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-11
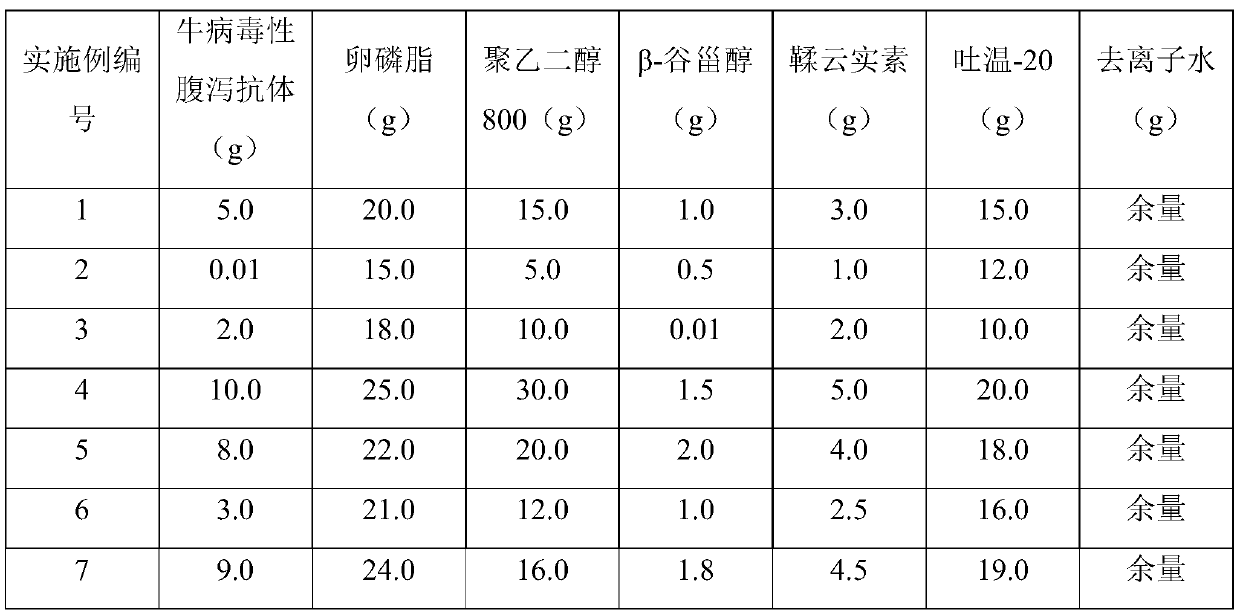
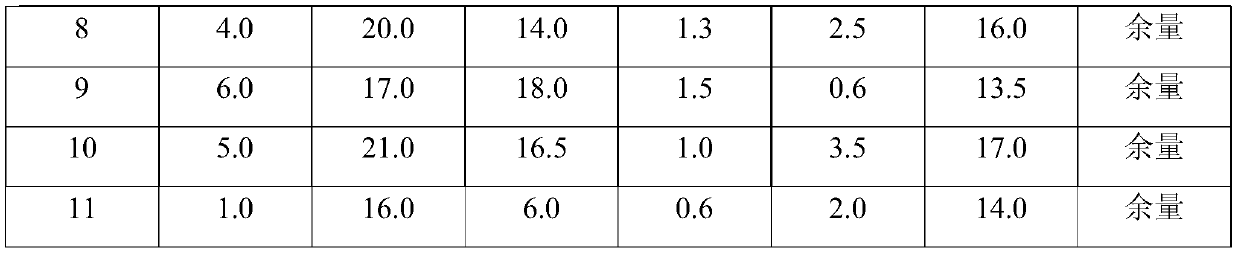
[0024] In order to make the description concise, the weight composition of the antibody preparations for preventing and treating bovine viral diarrhea described in Examples 1-11 is given below in the form of a table, as shown in Table 1 for details.
[0025] The weight composition per 100g of the antibody preparation of the present invention in Table 1 Examples 1-11
[0026]
[0027]
[0028] The preparation method of the antibody preparation for preventing and treating bovine viral diarrhea described in embodiment 1-11, comprises the following steps:
[0029] (a) Mix β-sitosterol and veil evenly and add to polyethylene glycol 800, stir until dissolved to obtain system 1;
[0030] (b) Mix bovine viral diarrhea antibody with lecithin, stir evenly, and obtain system 2;
[0031] (c) Mix System 1, System 2 and Tween-20, and stir evenly to obtain System 3;
[0032] (d) Heat system 3 to 35-45°C, add it to an emulsifier for emulsification, add the remaining amount of deionize...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com