Thermally-stable hepatitis A vaccine soluble microneedle patch and preparation method thereof
A thermally stable and soluble technology, applied in biochemical equipment and methods, microneedles, medical preparations of non-active ingredients, etc., to achieve moderate mechanical strength and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation method of hepatitis A vaccine soluble microneedle female mold
[0030] Such as figure 1 , the present invention adopts photolithography and etching process in conjunction with inverted mold method to prepare the negative mold of hepatitis A vaccine microneedle, and the steps are as follows:
[0031] 1) Preparation of monocrystalline silicon positive mold microneedles
[0032] The positive mold microneedles were prepared by photolithography and etching processes. The microneedles are listed as 5*5, the height of each microneedle is 400 μm, the diameter of the bottom of the needle is 200 μm, and the diameter of the tip is 20 μm.
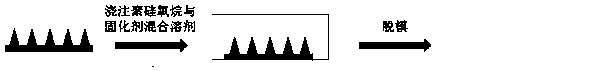
[0033] 2) Preparation of polysiloxane mold
[0034] Mix the polysiloxane and the curing agent at a mass ratio of 6:1, and pour it into a cuboid container with a single crystal silicon positive mold microneedle; place the container in a vacuum drying oven, and evacuate it for 3 minutes at a vacuum degree of 0.1MPa , so th...
Embodiment 2
[0035] The preparation of embodiment 2 hepatitis A vaccine microneedles
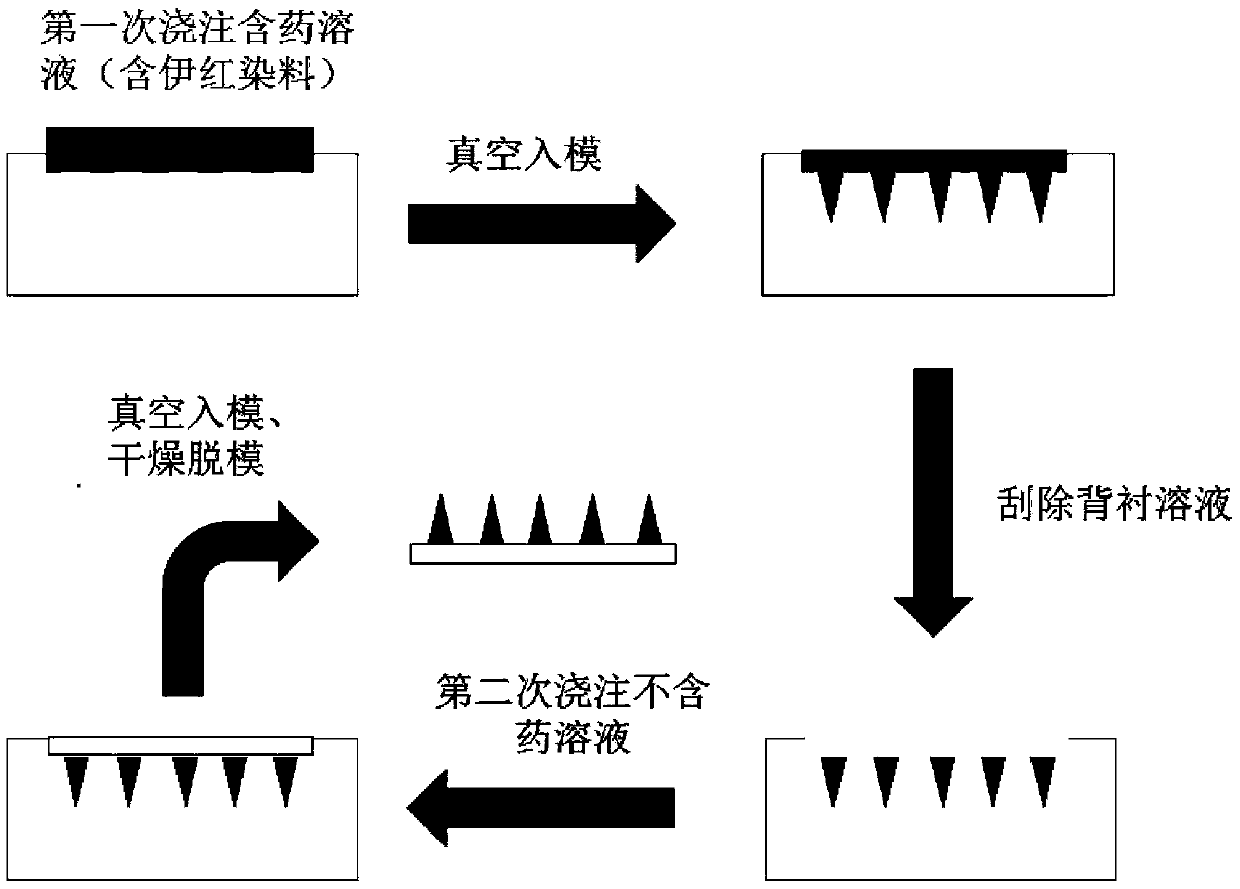
[0036] Such as figure 2 , the present invention adopts secondary vacuum molding method to prepare hepatitis A vaccine microneedles, and the steps are as follows:
[0037] 1) After mixing the matrix material, vaccine stabilizer and hepatitis A vaccine according to the prescription ratio, dissolve it with an appropriate amount of solvent (deionized water) to form a uniform mixture (i.e. needle body fluid), take an appropriate amount of needle body fluid into a centrifuge tube, and place in a centrifuge Precipitator, centrifuge to remove the air bubbles in the needle body fluid, and let it stand for later use.
[0038] 2) Take the needle body fluid obtained after centrifugation in step 1), pour it on the polysiloxane mold prepared in Embodiment 1 of the present invention, then place the polysiloxane mold in a vacuum drying oven, and put it in a vacuum degree of 0.1 MPa for 3 minutes, so that Inject the n...
Embodiment 3
[0041] The impact of embodiment 3 different vaccine stabilizers on the stability of hepatitis A vaccine
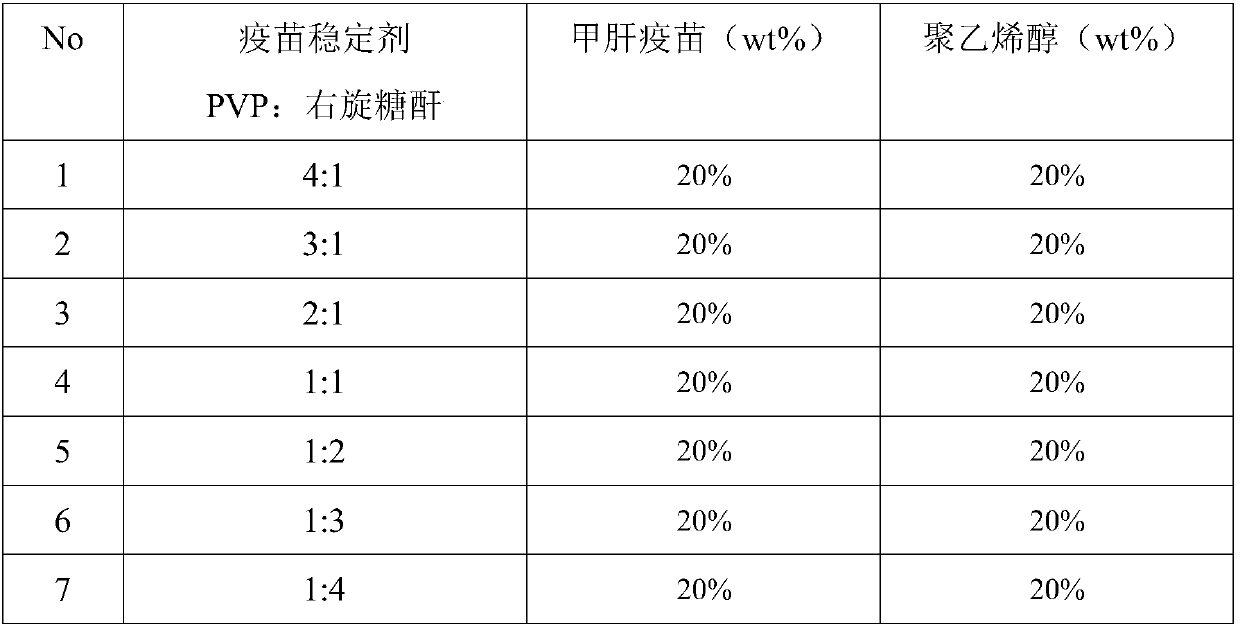
[0042] Assuming that the proportion of the vaccine stabilizer in the total mass of the needle is a certain value, only the components of the vaccine stabilizer are changed, and the effects of single or different combinations of the vaccine stabilizer on the stability of the hepatitis A vaccine in the microneedles are investigated. Influence, the preparation method of the microneedle and negative mold involved in this embodiment refers to Embodiment 1 and Embodiment 2 of the present invention.
[0043] Characterization method of vaccine stability in microneedles: the microneedles prepared according to the prescriptions in Table 1 were dissolved in 10 mL of deionized water at 37° C., and the vaccine antigen content in the solution was detected by ELISA (enzyme-linked immunosorbent assay). Record it as the vaccine antigen content in the initial month 0 (vaccine stability is r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com