Four-strain flu vaccine soluble microneedle patch and preparation method thereof
An influenza virus, soluble technology, applied in the field of quadrivalent influenza virus vaccine soluble microneedle patch and its preparation, can solve the mechanical properties of vaccine soluble microneedle, the difference in intradermal dissolution performance, the inability of microneedle tip to effectively pierce the skin, the physical and chemical properties of vaccine There are differences in properties, etc., to achieve the effects of good stability, fast onset, and moderate mechanical strength of the needle body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 4
[0036] Example 1 The negative mold preparation of quadrivalent influenza virus vaccine microneedles
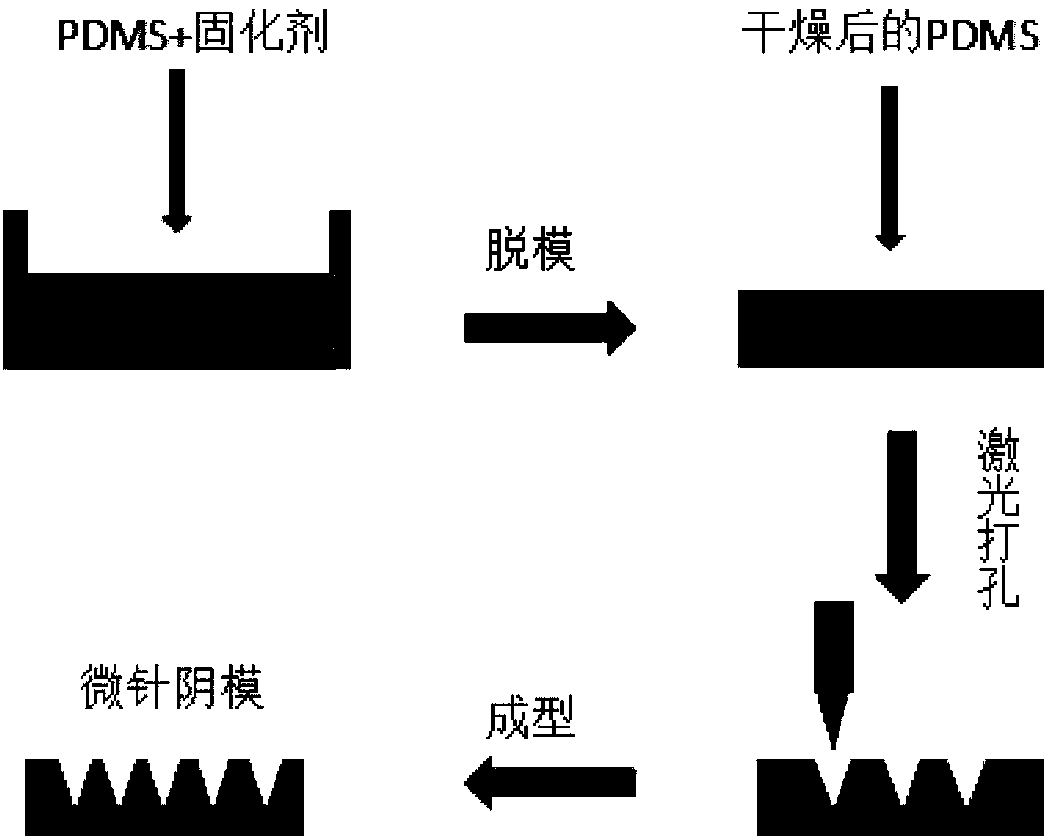
[0037] like figure 1 , the present invention adopts the laser drilling method to prepare the negative mold of the quadrivalent influenza virus vaccine microneedle, and the steps are as follows:
[0038] 1) Preparation of PDMS mold
[0039] After mixing the polydimethoxysiloxane (PDMS) and the curing agent in a mass ratio of 10:1, pour it into a rectangular parallelepiped container; place the container in a vacuum drying box, and vacuumize for 5 minutes at a vacuum degree of 0.08 MPa to make the mixing The air bubbles in the liquid are removed; then put in an oven, dried at 60° C. for 5-10 hours, and then taken out to obtain a formed PDMS mold.
[0040] 2) Laser drilling PDMS mold
[0041] Use a carbon dioxide laser engraving machine to drill holes on the PDMS mold obtained in step 1) to obtain a negative mold that can be used for preparing quadrivalent influenza virus vacci...
Embodiment 2 4
[0043] The preparation of embodiment 2 quadrivalent influenza virus vaccine microneedle
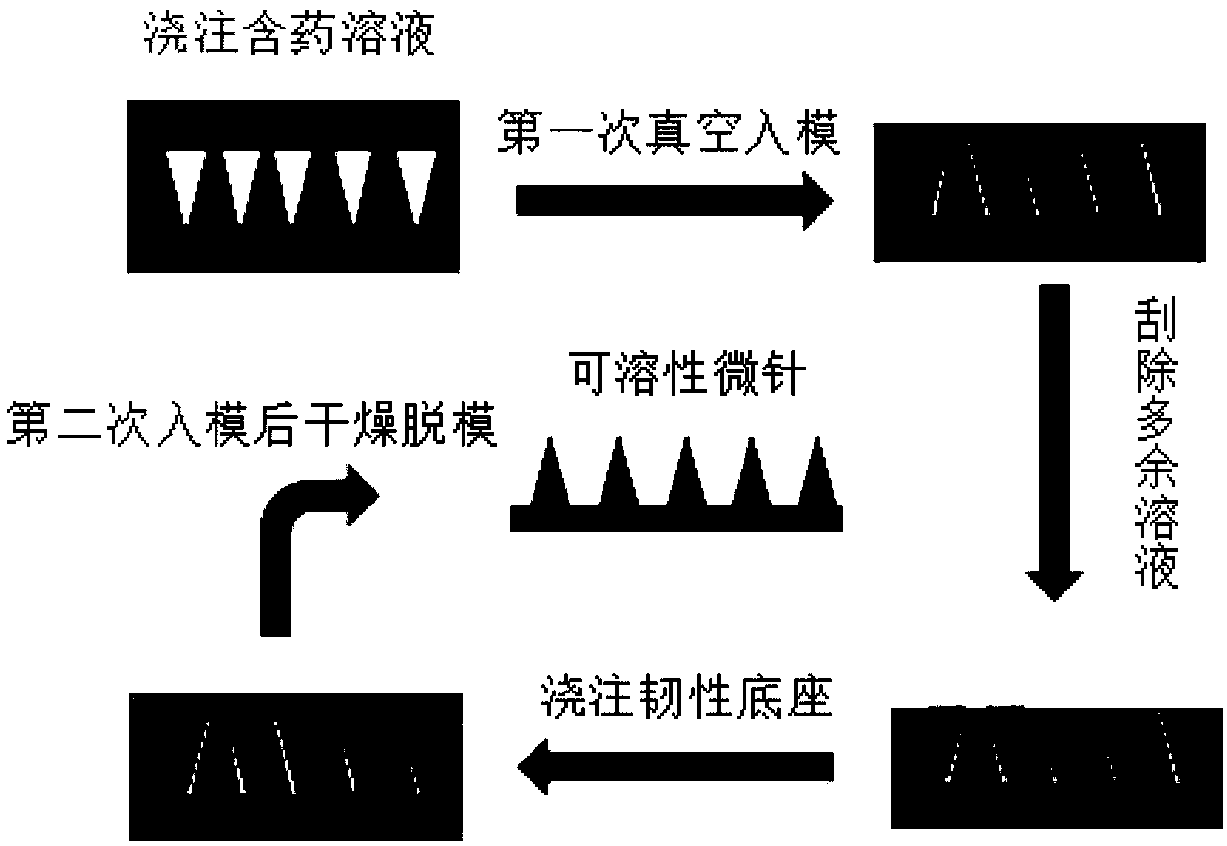
[0044] like figure 2 In the present invention, the quadrivalent influenza virus vaccine microneedle is prepared by a secondary vacuum mold-in method, and the steps are as follows:
[0045] 1) After mixing Gantrez S-97, CS, trehalose, and quadrivalent influenza virus vaccine according to the prescribed proportion, dissolve and mix with solvent (deionized water) respectively to form a uniform needle body fluid, take an appropriate amount of needle body fluid and put it into a centrifuge tube, Placed in a centrifugal precipitation device, centrifuged to remove air bubbles in the needle fluid, and set aside for later use.
[0046] 2) Take the needle body fluid obtained after centrifugation in step 1), pour it on the PDMS mold prepared in the implementation 1 of the present invention, and then place the PDMS mold in a vacuum drying box, and vacuumize for 5 minutes at a vacuum degree of 0.08 MP...
Embodiment 3
[0050] Example 3 Experiment on the influence of the components of the matrix material on the performance of the microneedles
[0051] Assuming that the proportion of the matrix material in the total mass of the needle body is a certain value, only the composition of the matrix material is changed, and the influence of different combinations of matrix materials on the performance of the microneedle as shown in Table 1 is investigated. The microneedle involved in this example For the negative mold, the preparation method thereof refers to Example 1 and Example 2 of the present invention.
[0052] Table 1 Microneedle formulations composed of different matrix material components
[0053] No Matrix material trehalose Quadrivalent influenza virus vaccine 1 40% PVP 15% 45% 2 40% Gantrez S-97 15% 45% 3 40% CS 15% 45% 4 40% PVA 15% 45% 5 20% PVP + 20% Gantrez S-97 15% 45% 6 20% CS+20% PVA 15% 45% 7 20% Gantrez S-97 +...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com