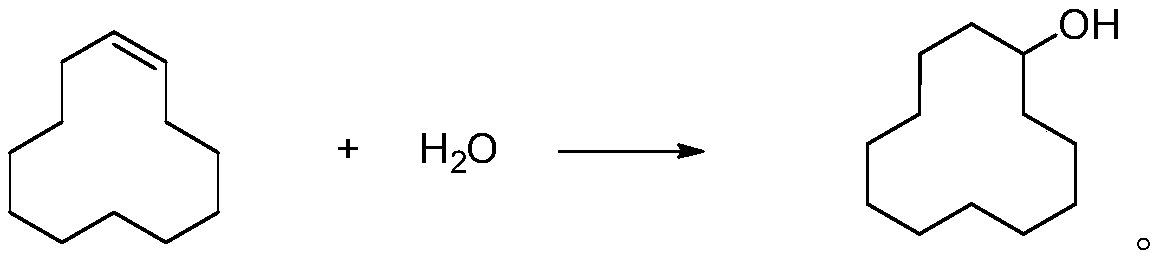
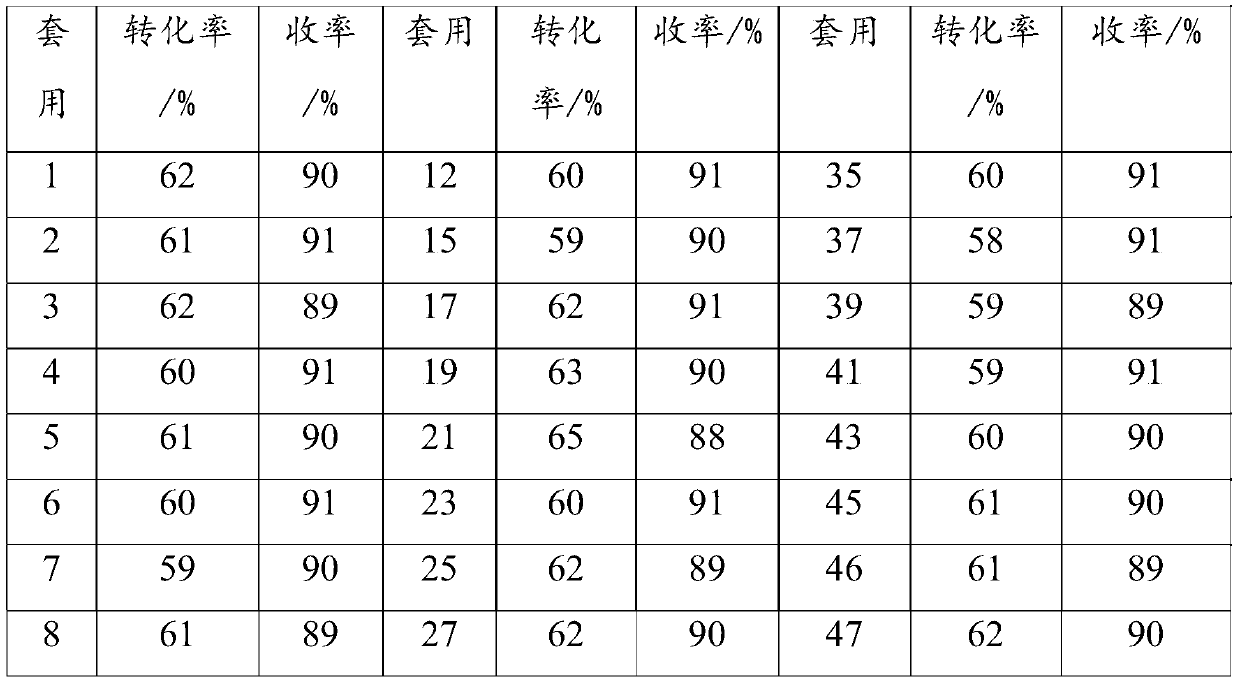
Cyclododecanol preparation method
A technology of cyclododecanol and cyclododecene, applied in the field of preparation of cyclododecanol, can solve problems such as difficulty in taking into account the utilization rate and selectivity of hydrogen peroxide in oxidation reaction, adverse effects of separation and purification, and explosion of peroxide, and the like. Conducive to safe operation, green reaction process, and the effect of promoting complete conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 3L autoclave, add 1.0Kg of cyclododecene, 100g of tert-butanol, 100g of water, 10g of H-ZSM-5, blow nitrogen gas at 0.01MPa, raise the temperature to 100°C, and monitor the reaction for 6 hours. The oil and water were separated, and then the unreacted cyclododecene and the crude product were removed under reduced pressure, and the rectification separation obtained cyclododecanol with a conversion rate of 45% (GC content of 99.5%) and a yield of 84%.
Embodiment 2
[0031] In a 50L high-pressure reactor, add 1.0Kg of cyclododecene, 10Kg of tert-amyl alcohol, 2Kg of water, 10Kg of H-β molecular sieves, feed argon gas at 10.0MPa, raise the temperature to 250°C, and monitor the reaction for 4 hours. Oil and water were separated, and then the unreacted cyclododecene and the crude product were decompressed to obtain cyclododecanol with a conversion rate of 75% (GC content of 99.5%) and a yield of 86%.
Embodiment 3
[0033] In a 5L high-pressure reactor, add 1.0Kg of cyclododecene, 1Kg of polyethylene glycol dimethyl ether, 500g of water, 100g of macroporous sulfonic acid resin, feed carbon monoxide at 1MPa, raise the temperature to 120°C, and maintain the reaction for 3 hours. After the reaction, the oil and water were separated, and then the unreacted cyclododecene and the crude product were removed under reduced pressure, and the rectification and separation obtained cyclododecanol with a conversion rate of 70% (GC content of 99.5%) and a yield of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com