Fluorine-containing AZD9291 derivative as well as preparation method and application thereof
A technology of AZD9291 and derivatives, applied in the field of fluorine-containing AZD9291 derivatives and their preparation, can solve problems such as poor metabolism, and achieve the effects of good inhibitory effect, strong in vitro anti-tumor activity, and enhanced in vitro anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
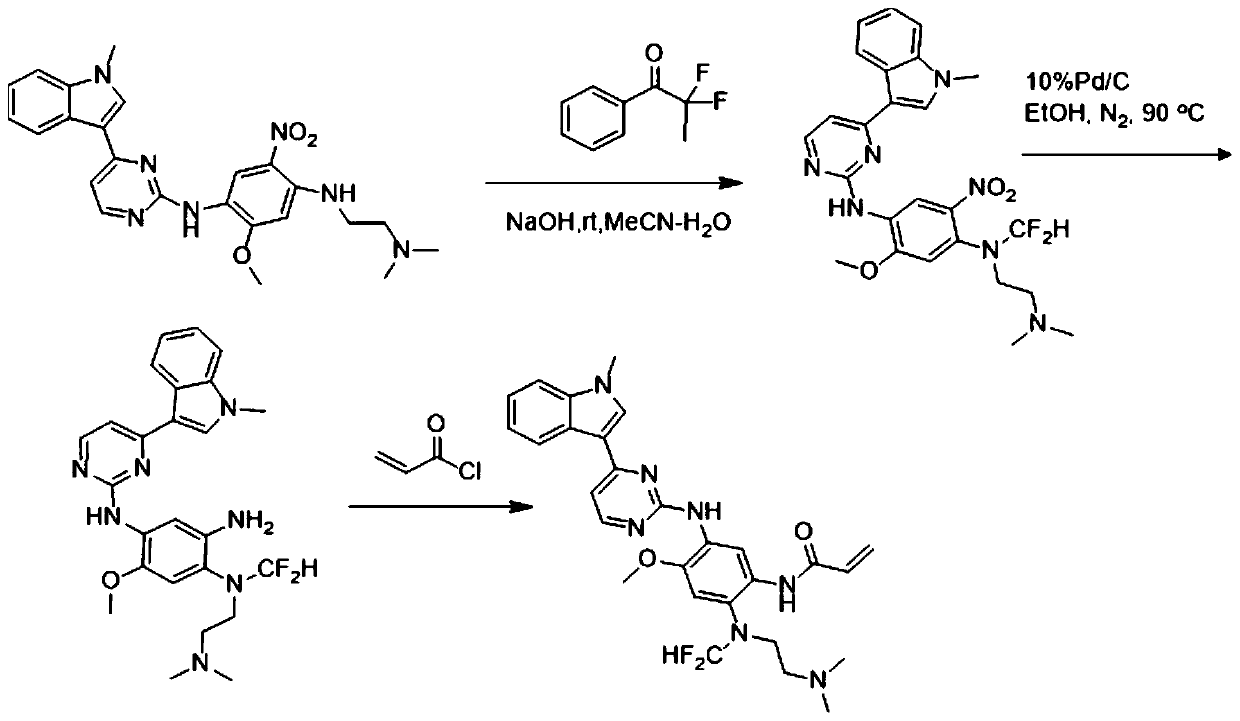
[0037] N-(2-((difluoromethyl)(2-(dimethylamino)ethyl)amino)-4-methoxy-5-((4-(1-methyl-1H-indole- The preparation of 3-yl) pyrimidin-2-yl) amino-yl) phenyl) acrylamide, that is, the AZD9291 derivative with structure a, is prepared by the following method, and the preparation route is as follows: figure 1 Shown:
[0038] Weigh compound N1-(2-(dimethylamino)ethyl)-5-methoxy-N 4-(4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl )-2-nitrobenzene-1,4-diamine (461mg, 1mmol) was placed in a 100ml eggplant-shaped bottle, acetonitrile and water 20:1 were added, then sodium hydroxide (440mg, 11mmol) was added, and finally 564mg ( 2mmol) 2,2-difluoro-2-iodo-1-phenylethan-1-one, react at room temperature for 15 minutes. After the reaction is completed, spin dry, add water, extract with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, concentrate, and undergo reduction and amidation. The reduction uses a Pb / C structure with a palladium ratio of 10% as a catalyst. Unde...
Embodiment 2
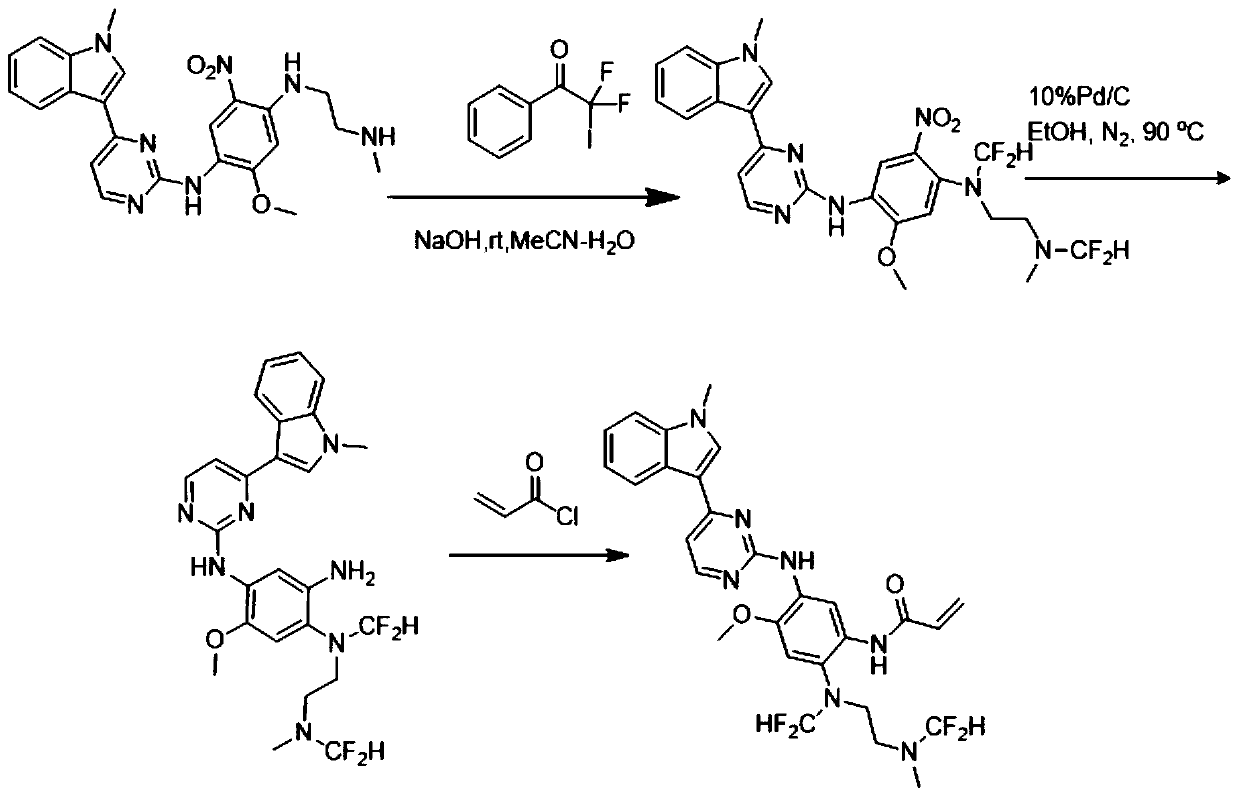
[0040] N-(2-((difluoromethyl)(2-((difluoromethyl)(methyl)amino)ethyl)amino)-4-methoxy-5-((4-(1-methyl The preparation of base-1H-indol-3-yl)pyrimidinepyridin-2-yl)amino)phenyl)acrylamide, that is, the AZD9291 derivative with structure b, is prepared by the following method, and the preparation route is as follows figure 2 Shown:
[0041]Weigh the compound 2-methoxy-N4-methyl-N1-(4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl)-N4-(2-(methylamino ) ethyl)-5-nitrobenzene-1,4-diamine (461mg, 1mmol) was placed in a 100ml eggplant-shaped bottle, added acetonitrile and water 20:1, then added sodium hydroxide (440mg, 11mmol), and finally Add 564 mg (2 mmol) of 2,2-difluoro-2-iodo-1-phenylethan-1-one and react at room temperature for 15 minutes. After the reaction is completed, spin dry, add water, extract with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, concentrate, undergo reduction and amidation, quench with saturated sodium bicarbonate solution, extra...
Embodiment 3
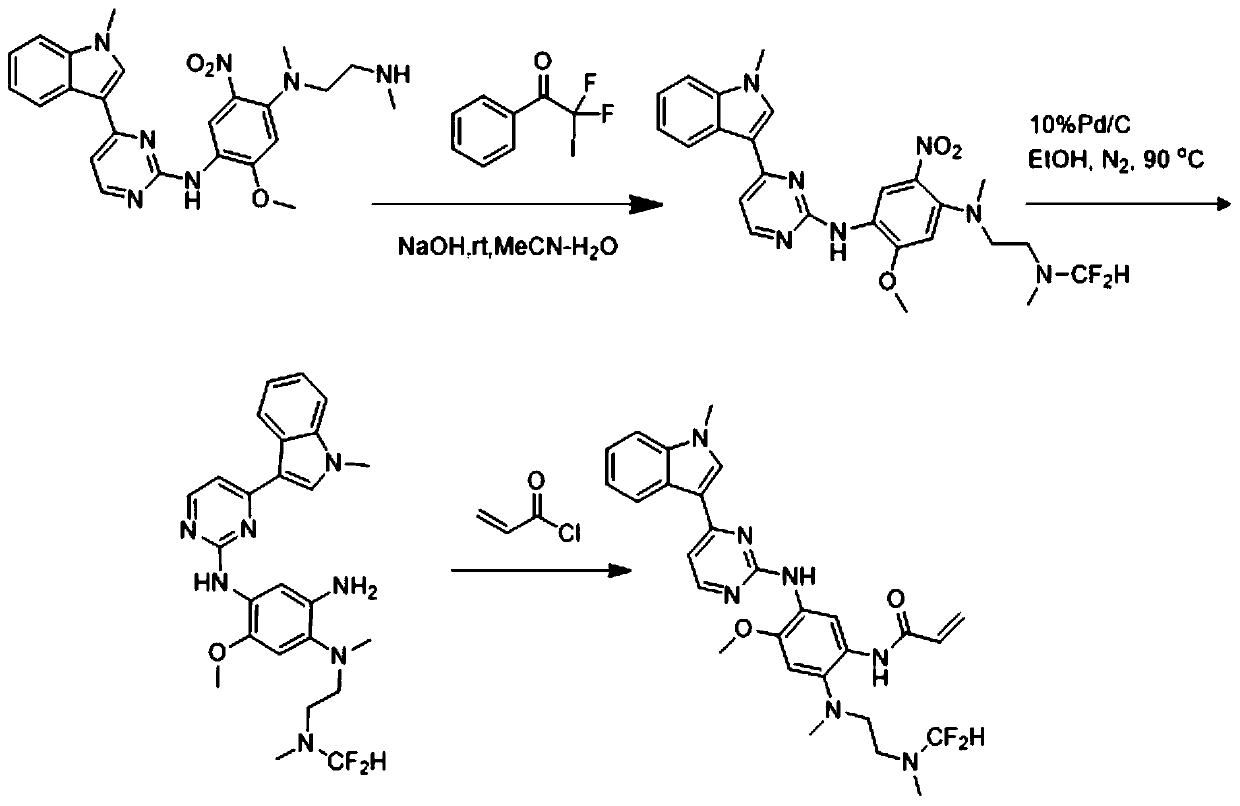
[0043] N-(2-((difluoromethyl)(2-((difluoromethyl)(methyl)amino)ethyl)amino)-4-methoxy-5-((4-(1-methyl The preparation of base-1H-indol-3-yl)pyrimidinepyridin-2-yl)amino)phenyl)acrylamide, that is, the AZD9291 derivative with structure c, is prepared by the following method, and the preparation route is as follows image 3 Shown:
[0044] Weigh compound 2-methoxy-N 1-(4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl)-N4-(2-(methylamino)ethyl) -5-nitrobenzene-1,4-diamine (447mg, 1mmol) was placed in a 100ml eggplant-shaped bottle, acetonitrile and water 20:1 were added, then sodium hydroxide (440mg, 11mmol) was added, and finally 564mg (2mmol ) 2,2-difluoro-2-iodo-1-phenylethan-1-one, react at room temperature for 15 minutes. After the reaction is completed, spin dry, add water, extract with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, concentrate, undergo reduction and amidation, quench with saturated sodium bicarbonate solution, extract with ethyl ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com