Coxsackie virus group A 16-type monoclonal antibody 16E1 and preparation method thereof
A monoclonal antibody and Coxsackie virus technology, applied in the field of antibody drugs and virology, can solve the problem of lack of CA16 antigen detection kits, etc., and achieve excellent application significance and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
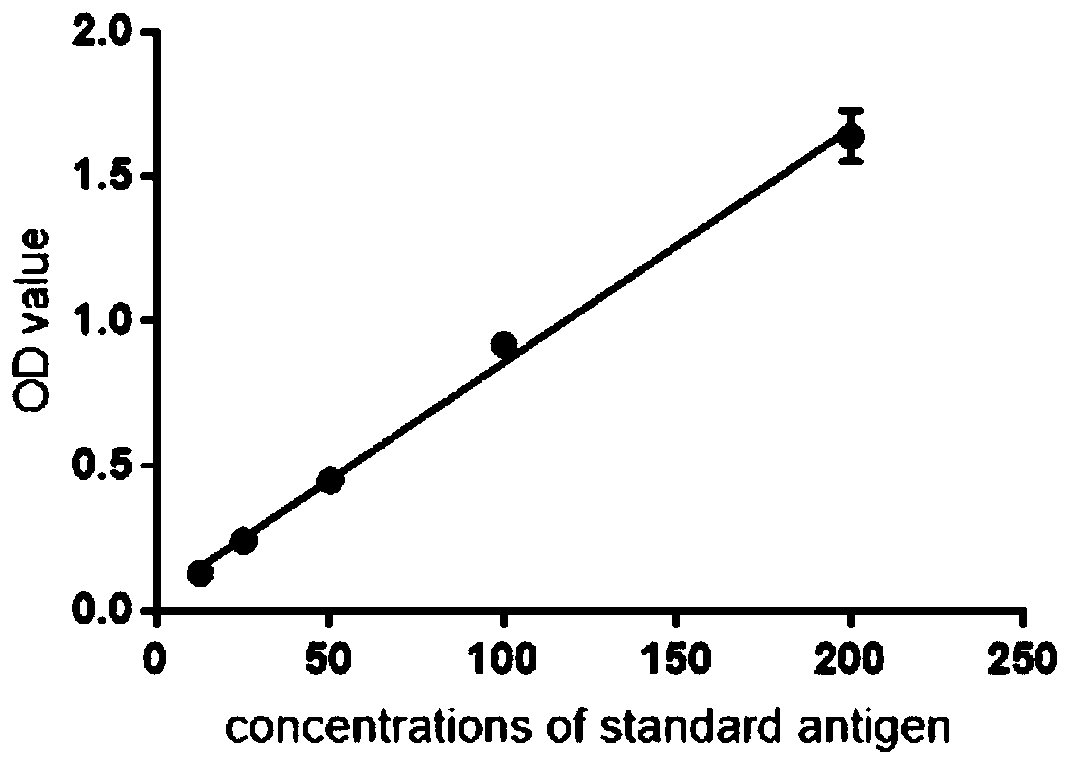
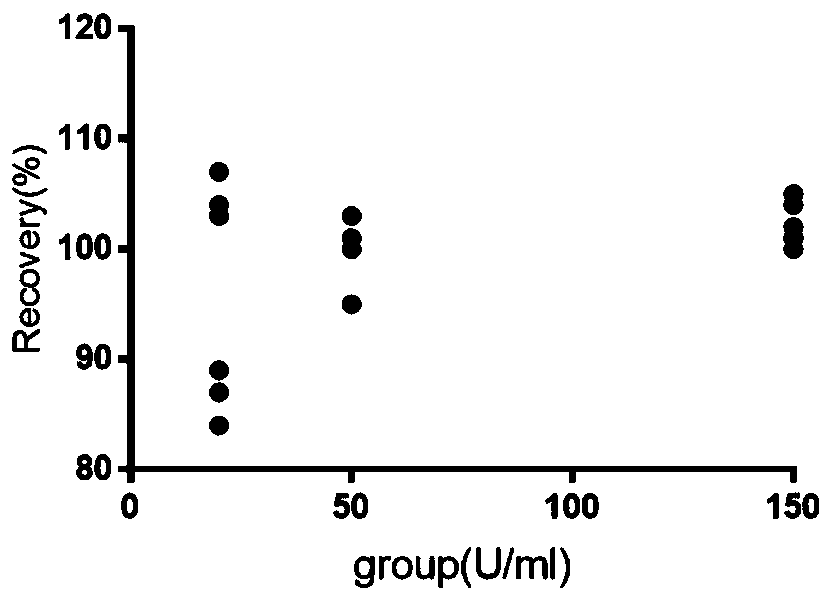
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of anti-CA16 monoclonal antibody
[0049] (1) Immunization of mice
[0050] Immunization of CA16 virus: BALB / c female mice aged 6-8 weeks were immunized with CA16 virus immunogen, and boosted immunization every 2 weeks. Before each immunization, 200 μL of eye socket vein blood or 20 μL of tail vein blood were collected for preparation Titer determination. When the serum titer of the mice reached the plateau, the immunization was stopped, and fusion was carried out after two months of rest.
[0051] (2) Preparation and screening of fusion hybridomas
[0052] 1. Preparation of mouse macrophages: A 6-week-old BALB / c mouse was killed, rinsed with tap water, and soaked in 75% ethanol solution for 5 minutes; the mouse was taken out and the abdomen was fully exposed, and sterile Lift the peritoneum with tweezers, cut a small opening in the center of the peritoneum, inject an appropriate amount of HT culture solution into the abdominal cavity through t...
Embodiment 3
[0071] Example 3: Preparation of CA16 HRP-labeled rabbit polyclonal antibody
[0072] The Vero cells were recovered and cultured at 36.5±0.5°C. The cell concentration reaches 0.1~10×10 6 Infection of Vero cells with CA16 virus (CA16V-24) at an MOI of 0.05 to 0.1, cultured at 35.5±0.5°C for 2 to 4 days, and the cell supernatant was harvested, which was the CA16 harvest solution. The CA16 harvest liquid was clarified and then concentrated more than 5 times by ultrafiltration membrane. Then carry out molecular sieve chromatography and ion exchange chromatography, the detection wavelength is 280nm, collect eluent and flow-through respectively to obtain purified solution, and obtain CA16 vaccine stock solution after formaldehyde inactivation. The purity of the CA16 purified liquid is over 90% as detected by HPLC. The detected antigen content was 13220U / ml, and the total protein content was 6μg / ml. Use this stock solution as the immunogen.
[0073] The immunized animals were 2...
Embodiment 4
[0083] Embodiment 4: Determination of antigen detection system
[0084] Three commonly used ELISA detection systems were compared, namely:
[0085] System 1: rabbit polyclonal antibody + antigen + 16E1 (mouse) + goat anti-rabbit HRP
[0086] System 2: 16E1 (mouse) + antigen + rabbit polyclonal antibody + goat anti-rabbit HRP
[0087] System 3: 16E1 (mouse) + antigen + rabbit polyclonal antibody HRP.
[0088] Adopt the checkerboard method to explore the appropriate concentration for use, and the antigen is the CA16 vaccine stock solution produced according to Example 1.
[0089] In the case of meeting the background requirements, the sensitivity of system 1 can only reach 125ng / ml, which cannot meet the detection requirements; the sensitivity of system 2 is the highest, reaching 1ng / ml; the sensitivity of system 3 can reach 12.5ng / ml. Since system 3 has fewer detection steps and can shorten the detection time, system 3 was chosen to establish the detection method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com