Hexaazabenzophenanthrene trianthraquinone derivative and synthesis method thereof
A technology of trianthracene and synthesis method, applied in the field of hexaazatriphenylene trianthracene derivatives and synthesis thereof, can solve the problems of insignificant improvement effect and the like, achieve mild and easy control of reaction conditions, improve electron withdrawing Ability, the effect of a short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
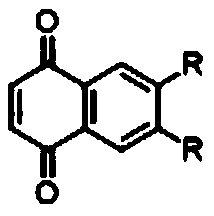
[0044] Compound A is 5,6-dodecyl-1,4-naphthoquinone, R is -C 12 h 23 .
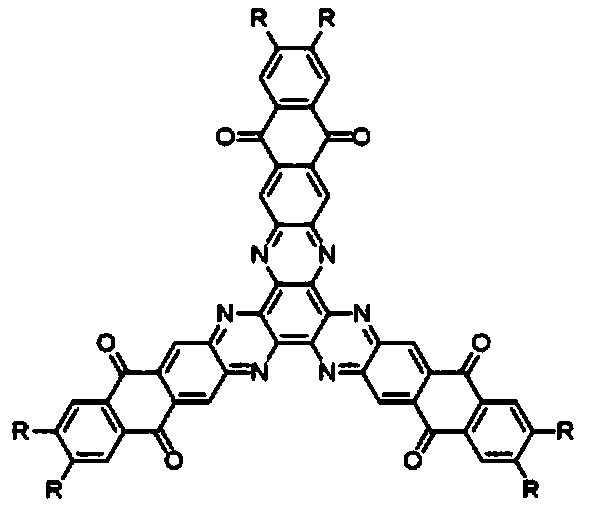
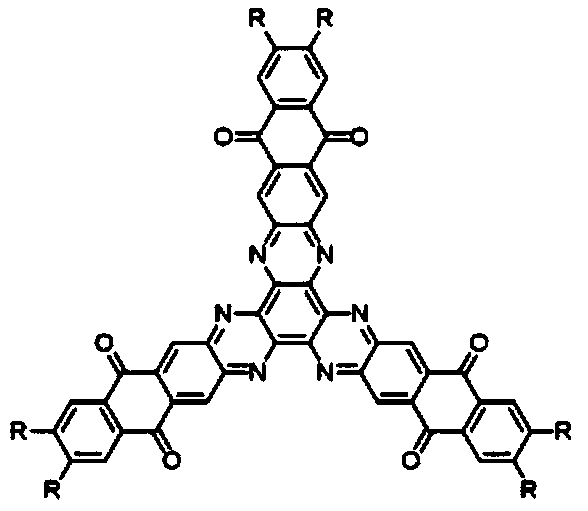
[0045] A hexaazatriphenylene trianthraquinone derivative, the structural formula of which is as follows:
[0046]
[0047] The synthetic method of above-mentioned hexaazatriphenanthrene trianthraquinone derivative specifically comprises the following steps:
[0048] (1) Synthesis of 2,3,6,7,10,11-hexa(dibromomethyl)-1,4,5,8,9,12-hexaazatriphenylene
[0049] Add 2.00g 2,3,6,7,10,11-hexamethyl-1,4,5,8,9,12-hexaazatriphenylene and 320mL CH 3 CN / H 2 O mixed solution (wherein CH 3 CN and H 2The volume ratio of O is 99:1), warming up to 85°C, stirring to 2,3,6,7,10,11-hexamethyl-1,4,5,8,9,12-hexaazabenzo After the phenanthrene is completely dissolved, slowly add 48 mL of Br in the reaction system 2 and 80mL CH 3 The mixed solution of CN was maintained at 85°C after the dropwise addition, and refluxed for 72 hours. Cool down to room temperature, add dropwise 300mL 0.50mol / LNaHSO 3 The solution was ...
Embodiment 2
[0053] Compound A is 5,6-bis(n-dodecyl ester group)-1,4-naphthoquinone, R is -COOC 12 h 23 .
[0054] A hexaazatriphenylene trianthraquinone derivative, the structural formula of which is as follows:
[0055]
[0056] Using the 2,3,6,7,10,11-hexa(dibromomethyl)-1,4,5,8,9,12-hexaazatriphenylene synthesized in Example 1 to synthesize the above-mentioned hexaaza Triphenylene trianthraquinone derivatives.
[0057] Vacuumize the 50mL three-neck flask-nitrogen for three cycles, add 0.96g 5,6-bis(n-dodecyl ester)-1,4-naphthoquinone, 0.63g to synthesize 2,3,6,7,10 , 11-hexa(dibromomethyl)-1,4,5,8,9,12-hexaazatriphenylene, 1.12g sodium iodide (NaI) and 20mL N,N-dimethylformamide (DMF ), heated to 90°C, and reacted for 48h under the protection of nitrogen. Cool to room temperature, mix the resulting mixture with 50 mL of acetone and stir for 30 min, filter, wash with deionized water and acetone until the filtrate is colorless, collect the filter cake, dry and column chromatograp...
Embodiment 3
[0059] Compound A is 5,6-bis(n-dodecylamide)-1,4-naphthoquinone, R is -CONHC 12 h 23 .
[0060] A hexaazatriphenylene trianthraquinone derivative, the structural formula of which is as follows:
[0061]
[0062] Using the 2,3,6,7,10,11-hexa(dibromomethyl)-1,4,5,8,9,12-hexaazatriphenylene synthesized in Example 1 to synthesize the above-mentioned hexaaza Triphenylene trianthraquinone derivatives.
[0063] The 50mL three-neck flask was evacuated-nitrogen for three cycles, and 0.95g of 5,6-bis(n-dodecylamide)-1,4-naphthoquinone, 0.63g of 2,3,6,7,10, 11-hexa(dibromomethyl)-1,4,5,8,9,12-hexaazatriphenylene, 1.24 g potassium iodide (KI) and 20 mL N,N-dimethylformamide (DMF), warming To 90 ° C, reacted under nitrogen protection for 48h. Cool to room temperature, mix the resulting mixture with 50 mL of acetone, stir for 30 min, filter, wash with deionized water and acetone until the filtrate is colorless, collect the filter cake, dry and column chromatography to obtain 0.57 g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com