Process for the preparation of lubiprostone and intermediates thereof
A technology for lubiprostone and compounds, applied in the field of preparing lubiprostone, capable of solving problems such as difficulty in lubiprostone purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] Preparation of Lubiprostone
[0028] The present invention provides a method for preparing lubiprostone, such as the reaction shown in the following scheme C:
[0029] Process C
[0030]
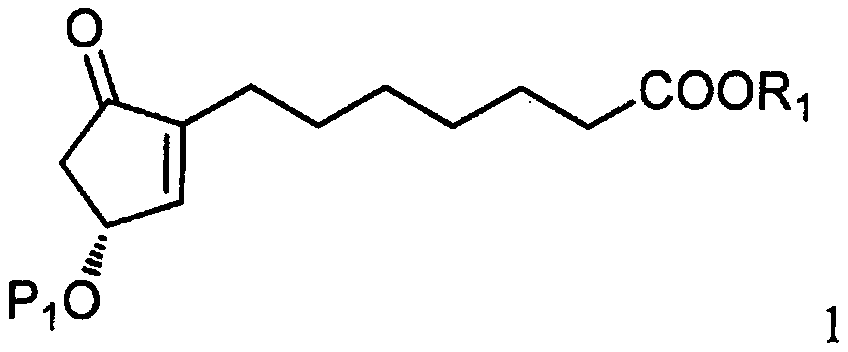
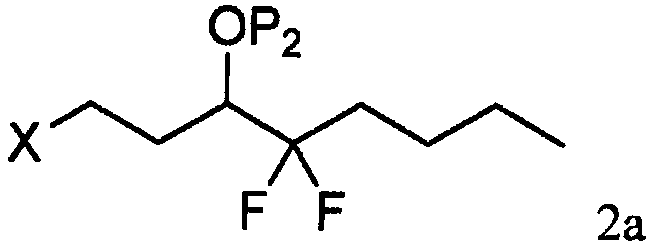
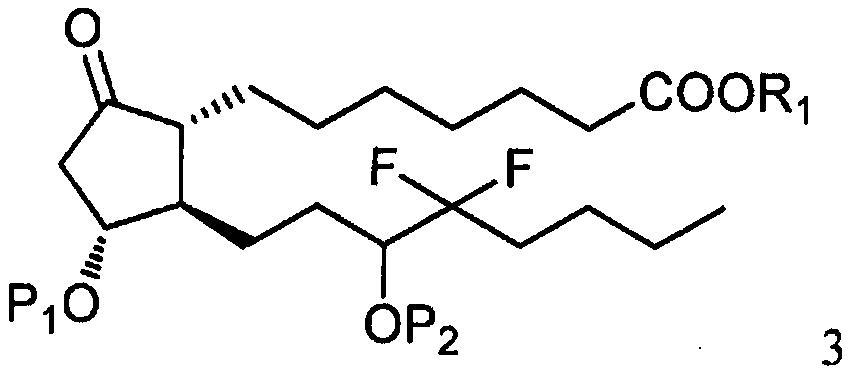
[0031] As shown in step a of scheme C, by the photoactive cyclopentenone of formula 1 (wherein R 1 is C 1-7 Alkyl, aryl or aralkyl, each of which is unsubstituted or C 1-4 Alkyl, nitro, halogen or alkoxy substituted, and P 1 is a hydroxyl protecting group) and a compound derived from formula 2 (wherein X is Cl, Br or I, and P 2 Be the coupling reaction of the ω-side chain unit of the cuprate of hydroxy protecting group or P) to prepare formula 3 compounds (wherein R 1 is C 1-7 Alkyl, aryl or aralkyl, each of which is unsubstituted or C 1-4 Alkyl, nitro, halogen or alkoxy substituted, and P 1 and P 2 independently a hydroxyl protecting group); the coupling reaction is preferably carried out at a temperature in the range of about -100°C to about 40°C.
[0032] Suitable hydr...
example 1
[0050] 4,4-Difluoro-3-keto-octanoic acid ethyl ester
[0051]
[0052] The 20 liter four-necked flask was flame dried and cooled under nitrogen. Diisopropylamine (585 g, 5.78 mol) dissolved in 4.7 L of anhydrous tetrahydrofuran was added to the reaction flask, followed by the dropwise addition of n-butyllithium (3.6 L, 1.6 M in hexane solution) and stirred for 1 hour. Next, ethyl acetate (509 g, 5.78 mol) was slowly added to the lithium solution. After 30 minutes, ethyl 2,2-difluorohexanoate (520 g, 2.89 mol) was added to the reaction flask at -70°C. The reaction mixture was stirred for 30 minutes and checked for reactivity using thin layer chromatography (TLC). The mixture was quenched with 5 L of saturated aqueous ammonium chloride and stirred for 10 minutes. The mixture was phase separated and the aqueous layer was extracted with 1 L of toluene. The organic layer was dried over anhydrous magnesium sulfate and the solid was filtered. The solvent was evaporated under...
example 2
[0056] 4,4-Difluorooctane-1,3-diol
[0057]
[0058] Sodium borohydride (253 g, 6.69 mol) was added to dissolve the crude ester product of Example 1 (875 g) in 4.5 L of ethanol at ambient temperature. The reaction mixture was stirred for 2 hours and checked for reactivity using TLC. Next, the reaction mixture was adjusted to a neutral solution with 3N aqueous hydrochloric acid solution. The aqueous solution was concentrated and ethanol was removed. The aqueous solution was extracted twice with 2.5 L of ethyl acetate. The organic layer was concentrated under vacuum. The crude 1,3-diol product was purified by silica gel chromatography using a mixture of hexane and ethyl acetate as a gradient eluent. The yield of 1,3-diol compound was 306 g (58%, two steps).
[0059] 1 H-NMR (CDCl 3 ): δ3.760~3.893(m, 5H), 1.678~1.922(m, 4H), 1.291~1.505(brs, 4H), 0.897(t, 3H)
[0060] 13 C-NMR (CDCl 3 ): δ123.997(t), 71.396(t), 60.040, 32.040(t), 31.561, 23.379(t), 22.461, 13.777
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com