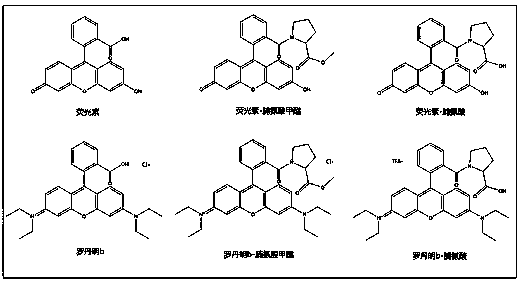
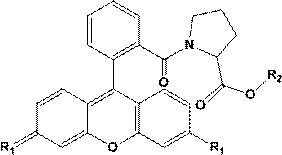
Easy-to-combine xanthene fluorescent compound as well as preparation and application thereof
The technology of a kind of compound and general formula compound is applied in the fields of easy-to-combine xanthene-based fluorescent compounds and their preparation and application, which can solve the problems of influence on fluorescent properties, destruction of conjugated system, high production cost, etc., to reduce production cost, reduce Hazards to persons and the environment, effects of good fluorescent properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
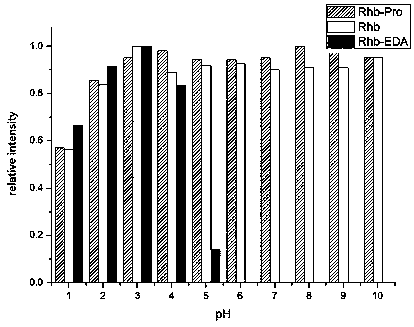
[0023] Embodiment 1. Synthesis of fluorescein-proline methyl ester
[0024] Dissolve 333mg fluorescein and 1mmol DIEA HBTU HOBt in 20ml DMF, cool down to 5°C for 15 minutes; dissolve 1mmol proline methyl ester hydrochloride and 1mmol DIEA in 10ml DMF and stir, then add the previous solution and react at room temperature for 10h. After the reaction was completed, 200ml of water was added, and extracted twice with 100ml of ethyl acetate. The organic phases were combined, washed twice with water, dried over anhydrous sodium sulfate, and separated by column chromatography to obtain 273 mg of the product, with a yield of 61.5%.
[0025] Chemical formula: C 26 h 21 NO 6 . Electrospray ionization mass spectrometry: [M+H] + =443.17.
Embodiment 2
[0026] Example 2. Fluorescein-proline synthesis
[0027] In Example 1, 444 mg of the product was dissolved in 10 ml of THF, 4 eq LiOH and 10 ml of water were added, and the reaction was stirred for 3 h. After the reaction was completed, the THF was spun off, 50ml of water was added, and the pH was adjusted to 3 with 1M hydrochloric acid. Solids were precipitated and filtered. Rinse twice with petroleum ether, filter and dry to obtain 335 mg of the product with a yield of 78%.
[0028] Chemical formula: C 25 h 19 NO 6 . Electrospray ionization mass spectrometry: [M+H] + =430.16. 1H NMR (400 MHz, CDCl3): δ 7.78 – 7.59 (m, 3H), 7.44 – 7.38 (m, 1H), 7.37 – 7.32 (m, 1H), 7.24 (dd, J= 10.0, 7.9 Hz , 2H), 6.88 – 6.77 (m, 2H), 6.72 – 6.66 (m, 1H), 4.39 (dd, J =8.6, 4.7 Hz, 1H), 3.31 (ddd, J = 19.8, 14.1, 5.4 Hz, 1H ), 3.26 – 3.14 (m,1H), 2.21 – 2.07 (m, 1H), 1.93 (ddd, J = 40.5, 21.0, 12.0 Hz, 1H), 1.75 (d, J= 4.8 Hz, 2H).
Embodiment 3
[0029] Example 3. Fluorescein-proline NHS ester
[0030] 525 mg of the product in Example 2 was dissolved in 40 ml of THF, an equivalent amount of DCC and NHS were added, and the reaction was stirred for 3 h. After the reaction, the insoluble matter was removed by filtration, and then the filtrate was spin-dried under low pressure, and the obtained solid was recrystallized with ethanol to obtain 360 mg of pure product with a yield of 68.4%. Chemical formula: C 29 h 22 N 2 o 8 . Electrospray ionization mass spectrometry: [M+H] + =526.17.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com