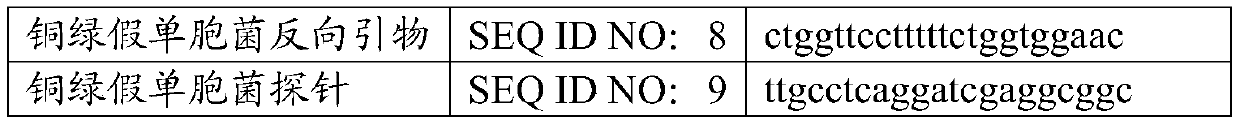Kit for detecting bloodstream infectious pathogen nucleic acids in plasma
A free nucleic acid and pathogen technology, which is applied in the field of kits for detecting free nucleic acid of bloodstream infection pathogens in plasma by using QPCR technology, and a kit for detecting free nucleic acid of bloodstream infection pathogens in plasma, which can solve the problem of low content of pathogenic microorganisms and restricted products. Application, influence sensitivity and other issues, to achieve the effect of high sensitivity, low solution efficiency, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Extraction of bloodstream infection pathogen free nucleic acid in the plasma sample of embodiment 1
[0032] Use a sterile 5ml centrifuge tube to collect 1.2ml of K2EDTA-treated human plasma samples, and use QIAamp Circulating Nucleic Acid Kit from QIAAMP for extraction. The operation steps are carried out according to the instructions. After extraction, the nucleic acid is placed at -75°C Save the following for later use.
Embodiment 2
[0033] Embodiment 2, primer, probe optimization experiment
[0034] Because cell-free DNA is all short fragments, the abundance of different target sequences in plasma varies. Different target gene sequences were designed for Klebsiella pneumoniae, Aspergillus flavus, and Pseudomonas aeruginosa, and the lengths of amplified fragments were different. The plasma of positive patients was used to extract free DNA and screen primers and probes.
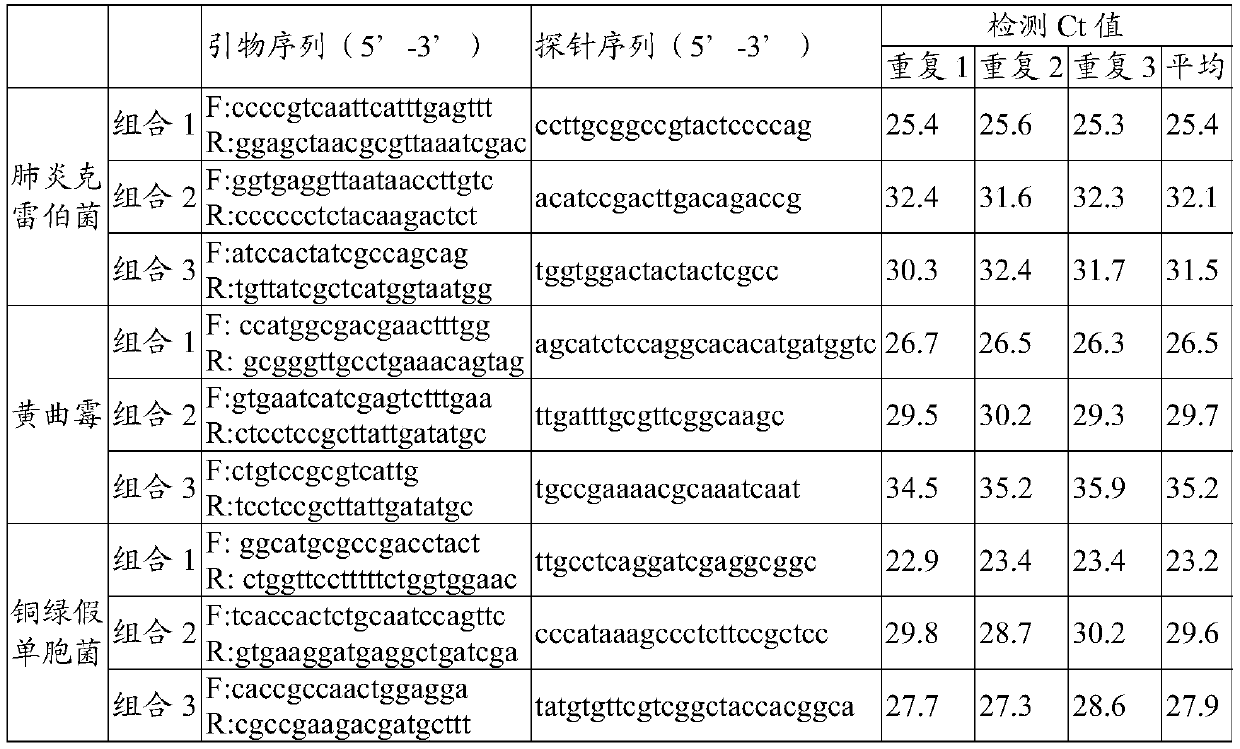
[0035] Table 1 Screening sequences of primers and probes
[0036]
[0037] For the same sample, the smaller the detection Ct value, the higher the detection sensitivity of the primer and probe combination. According to the results in Table 1, highly sensitive primers and probes were determined, and their sequences are shown in Table 2.
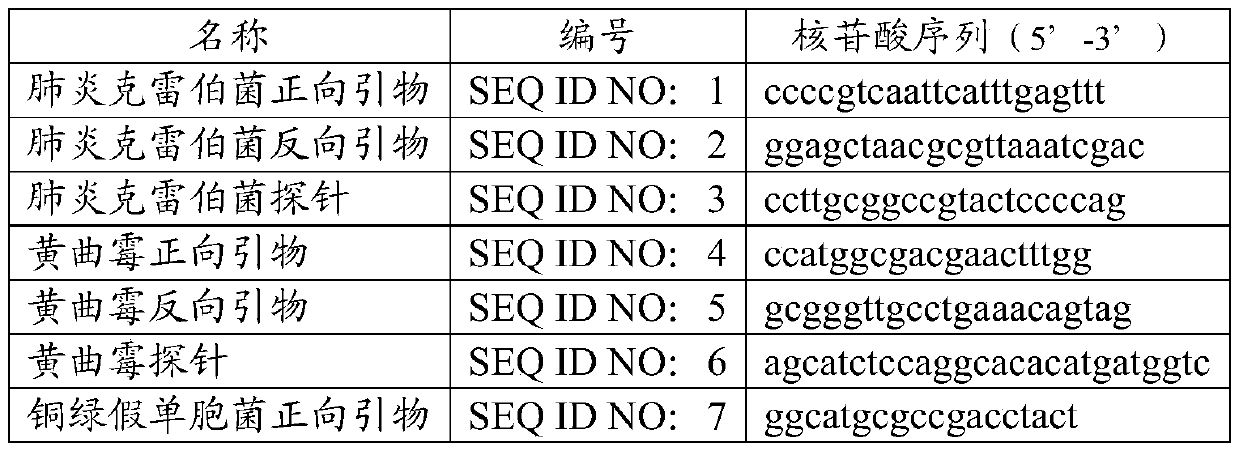
[0038] Table 2 highly sensitive primers and probe sequences
[0039]
[0040]
Embodiment 3
[0041] Embodiment 3, the preparation of Klebsiella pneumoniae, Aspergillus flavus, Pseudomonas aeruginosa triple nucleic acid detection kit
[0042] The primers and probe sequences in the kit are shown in Table 2.
[0043] The kit also includes internal standard primers and internal standard probes, the sequences of which are as follows:
[0044] Internal standard forward primer: 5'-3'gatgaagttggtggtgaggcc (SEQ ID NO: 11);
[0045] Internal standard reverse primer: 5'-3'tgcccagtttctattggtctcc (SEQ ID NO: 12);
[0046] Internal standard probe: 5'-3'gttccaccagaaaaaggaaccagacagg (SEQ ID NO: 13);
[0047] The kit also includes: 10mM dNTPs, 5U / μl Taq enzyme, 50mM MgCl 2 . The kit also includes a negative control (sterile water) and a positive control (artificially synthesized at a concentration of 1×10 6 Copies / ml of pseudovirus).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com