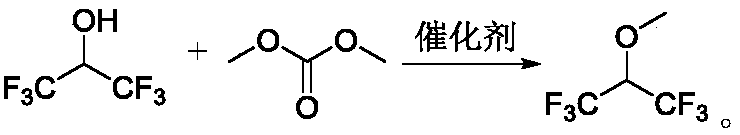
Method for preparing 1,1,1,3,3,3-hexafluoroisopropyl methyl ether through gas-phase methylation reaction
A technology of hexafluoroisopropyl methyl ether and hexafluoroisopropyl alcohol, applied in the field of gas-phase methylation reaction to prepare 1,1,1,3,3,3-hexafluoroisopropyl methyl ether, capable of Solve problems such as high cost, non-green chemical industry, and high energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
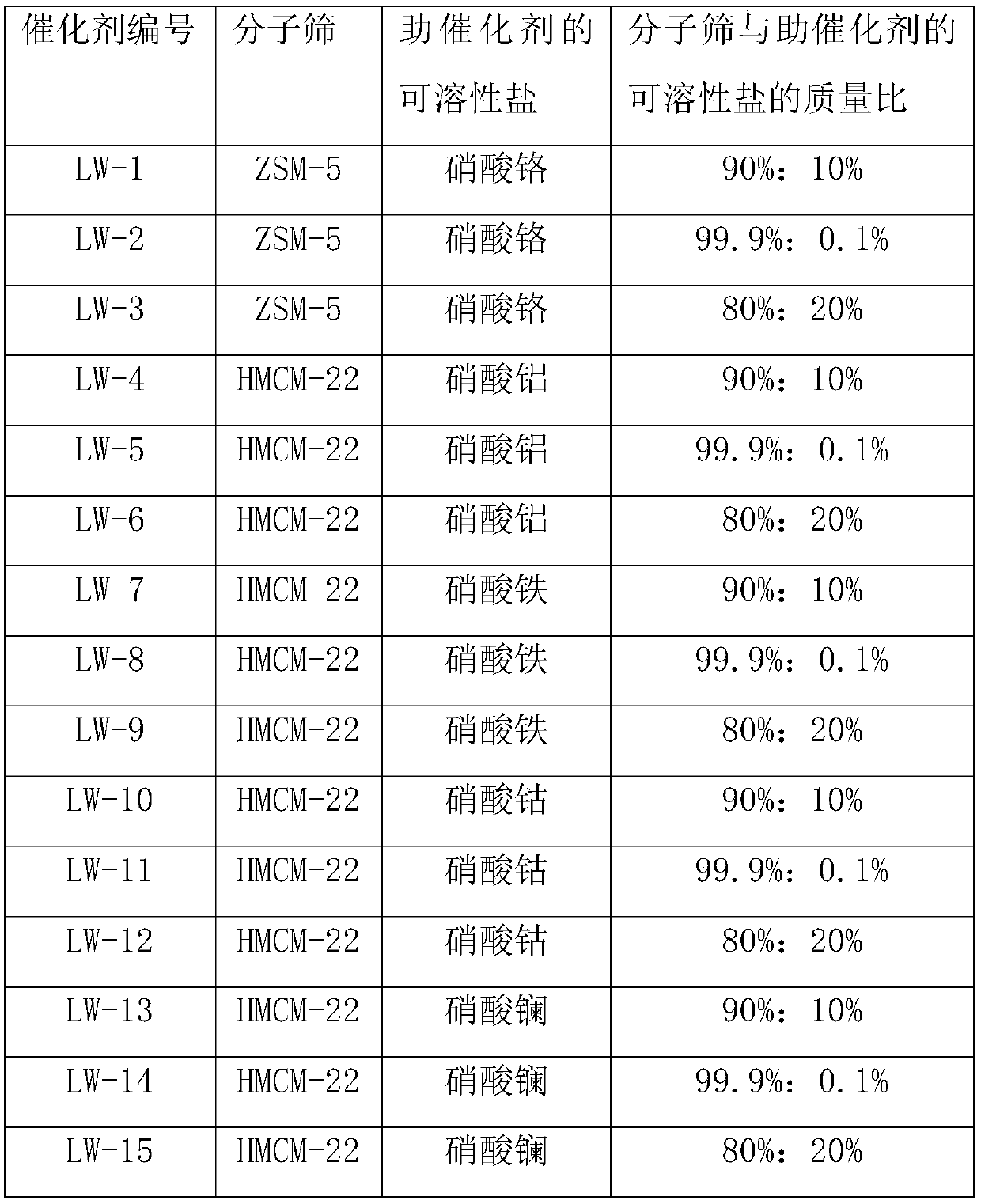
[0045] Embodiment 1, the preparation of methylation catalyst
[0046] Get chromium nitrate 23.8g to be dissolved in 500ml distilled water, then add 52g ZSM-5 molecular sieve and carry out impregnation, now the mass ratio of main catalyst ZSM-5 molecular sieve and cocatalyst is 90%: 10%, impregnation temperature is 50 ℃, and impregnation time is After 10 hours, it was filtered, dried, pulverized, and pressed to form a catalyst precursor. The catalyst precursor was calcined at 400° C. for 10 hours in an inert gas atmosphere to obtain a methylation catalyst, coded as LW-1.
[0047] According to the preparation method described in this example, the soluble salt of the molecular sieve and the cocatalyst was changed to prepare the catalyst described in the following table 1:
[0048] Table 1
[0049]
Embodiment 2
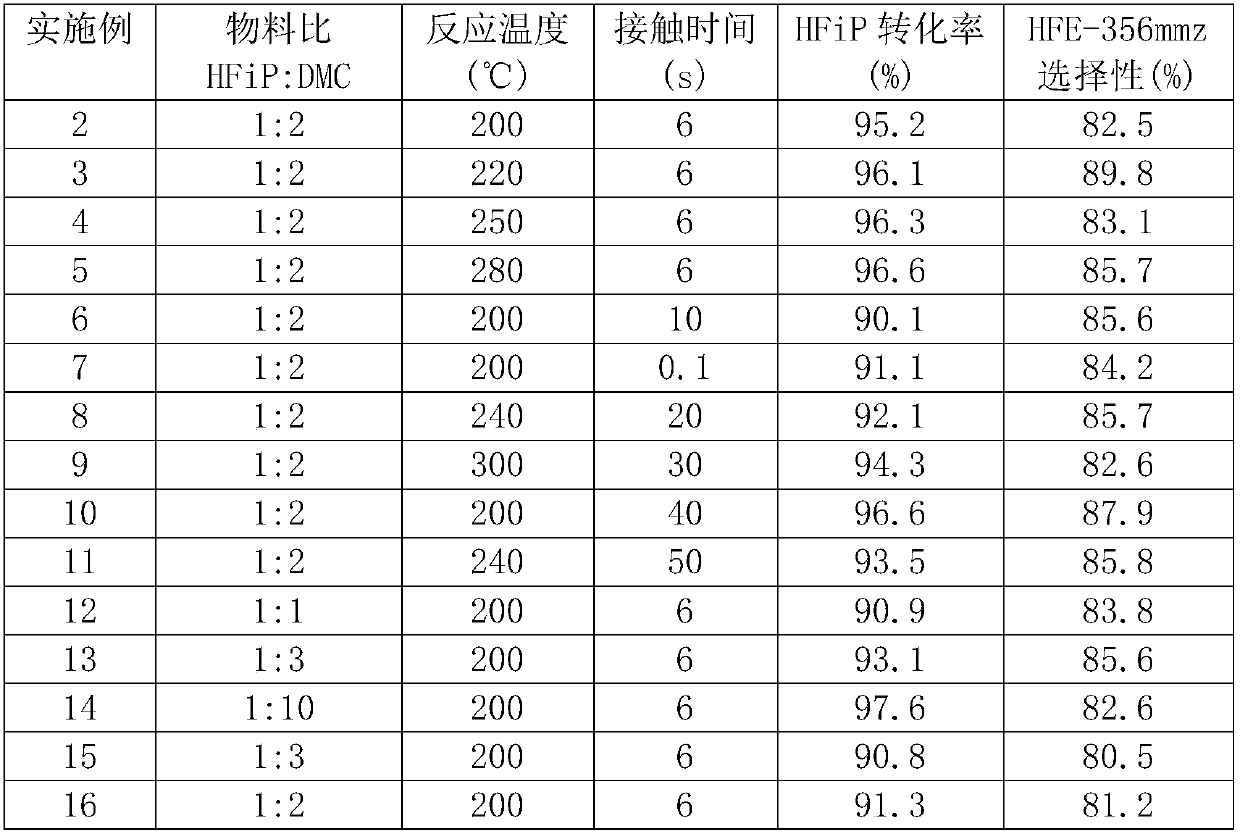
[0051] Fill 20ml gas phase methylation catalyst LW-1 in the tubular reactor made of Monel alloy with an inner diameter of 1 / 2 inch and a length of 40cm, the reactor is heated to 200°C, and the liquid raw material mixture is passed into After the preheating is completed, in the mixing chamber, the molar ratio of 1,1,1,3,3,3-hexafluoroisopropanol and dimethyl carbonate is controlled to be 1:2, and the liquid raw material is vaporized in the mixing chamber and passed through the catalyst The reactor, the contact time is 6 seconds, the reaction product is collected in the cold trap after cooling. The composition of the collected product was analyzed with a gas chromatograph, and the reaction results are listed in Table 2.
Embodiment 3
[0053]Inner diameter is 1 / 2 inch, and the tubular reactor made of Monel alloy that length is 40cm is filled with 20ml gas-phase methylation catalyst LW-2, and the reactor is heated up to 220 ℃, and the liquid raw material mixture is passed into by using a sampling pump. After the preheating is completed, in the mixing chamber, the molar ratio of 1,1,1,3,3,3-hexafluoroisopropanol and dimethyl carbonate is controlled to be 1:2, and the liquid raw material is vaporized in the mixing chamber and passed through the catalyst The reactor, the contact time is 6 seconds, the reaction product is collected in the cold trap after cooling. The composition of the collected product was analyzed with a gas chromatograph, and the reaction results are listed in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com