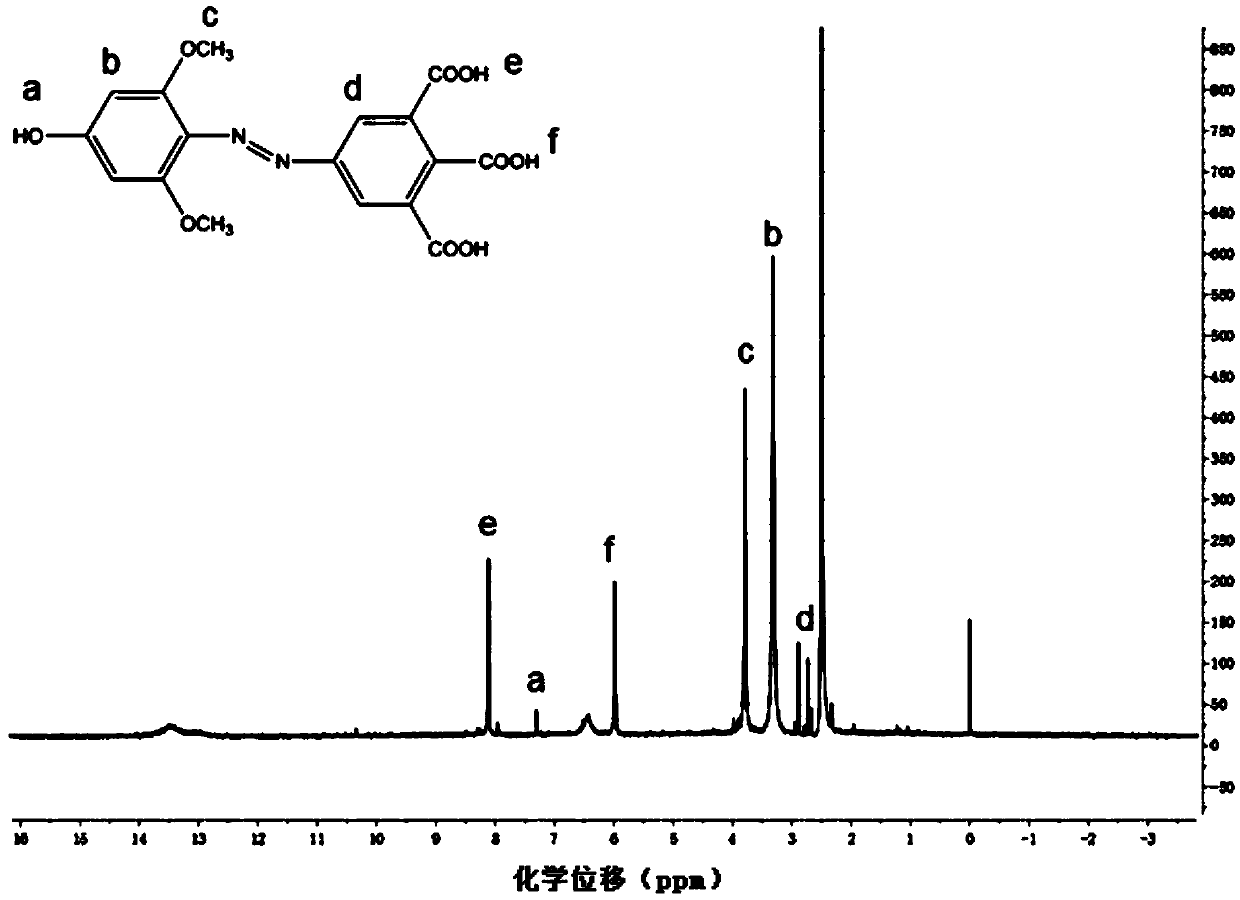
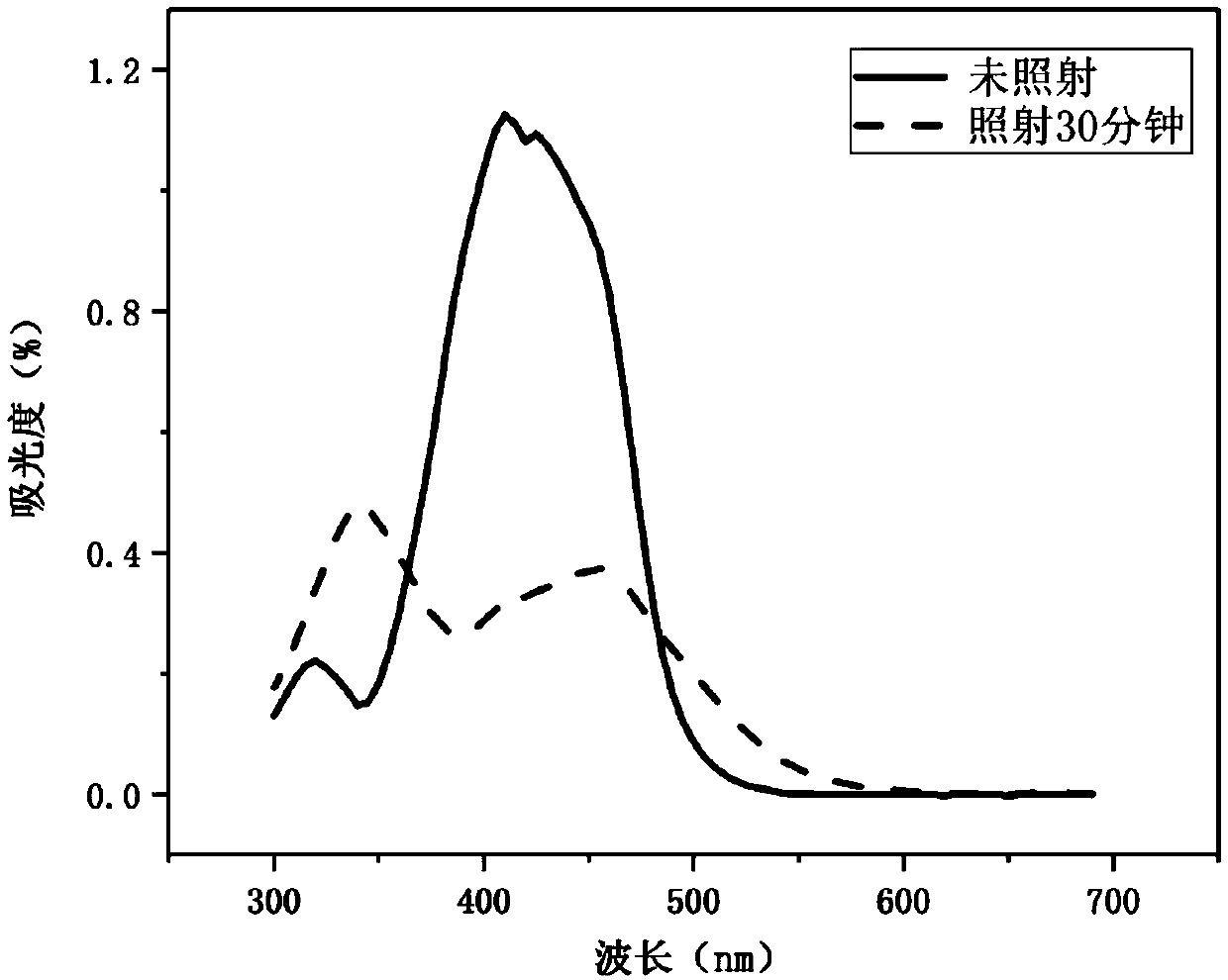
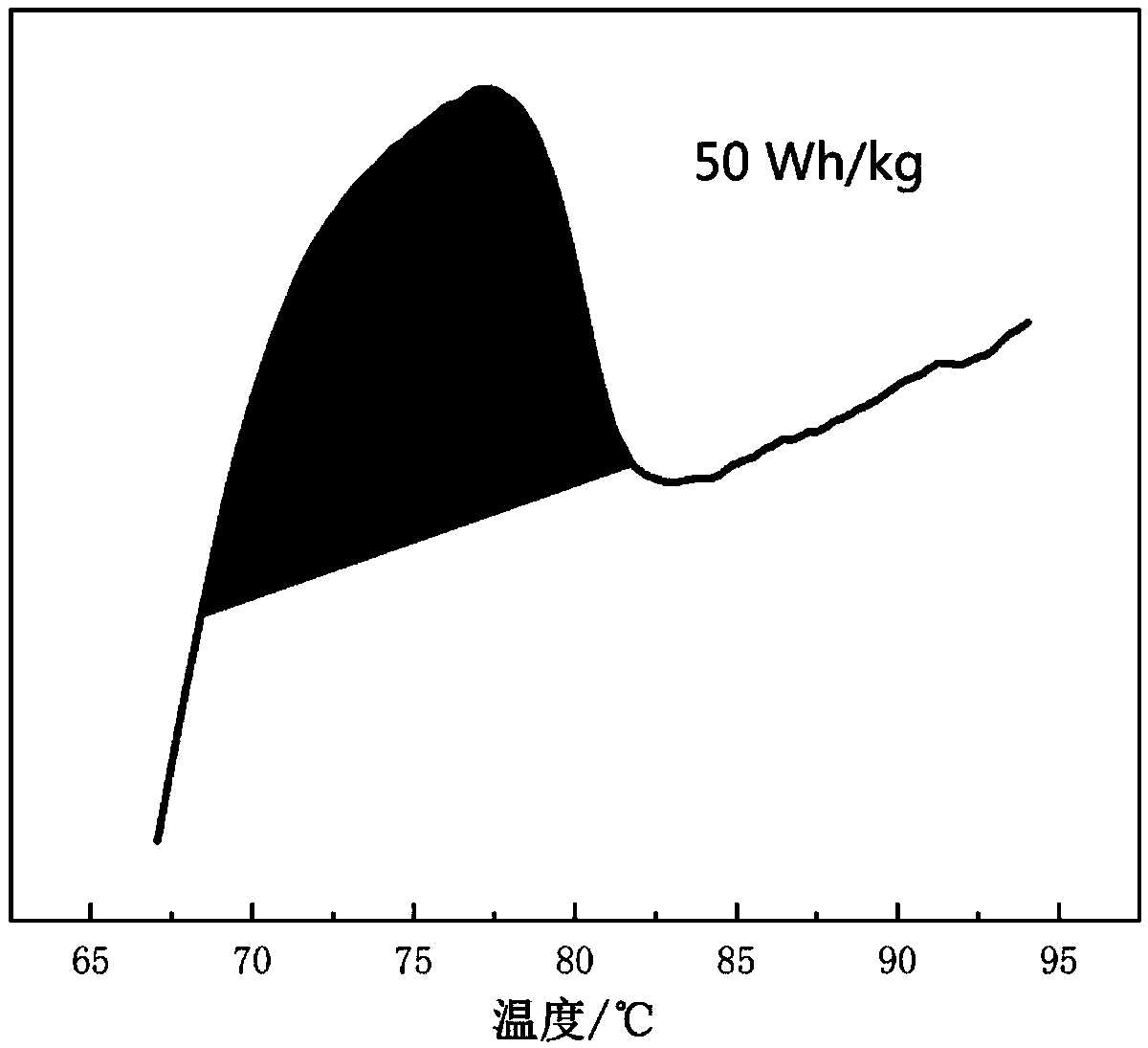
Azobenzene side chain polymerizable monomer based on modified cis-5-norbornene-2,3-dicarboxylic anhydride and applications thereof
A technology of norbornene and dicarboxylic anhydride, which is applied in the fields of polymer materials and solar energy storage, can solve the problems of low energy density and short half-life of azobenzene, and achieve the effects of high atom utilization rate, easy operation and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) The preparation of azobenzene (AZO) monomer is based on the mole of tricarboxyaniline, equivalent, 1.5 equivalents, 3 equivalents, and 4 equivalents are one time, 1.5 times, 3 times and 4 times of tricarboxyaniline.
[0040] ① 20mmol tricarboxyaniline was added to the aqueous solution in which 3 equivalents of sodium hydroxide were dissolved.
[0041] ②Dissolve an equivalent amount of sodium nitrite in water, and then add this solution dropwise to the solution in ① at 0°C under stirring conditions. After completely dissolving, add 4 equivalents of 1mol / L hydrochloric acid (that is, aqueous hydrogen chloride solution), Stirring was continued for 2 hours.
[0042] ③Under the protection of argon, 1.5 equivalents of 3,5-dimethoxyaniline was added to the solution of ②, stirred and reacted in ice bath for 5 hours, and AZO monomer was obtained.
[0043] 2) Preparation of polymer composites containing azo side chains:
[0044] ①Dissolve 20mmol of 6-amino-hexanol in concent...
Embodiment 2
[0048] 1) The preparation of AZO monomer, based on the mole of tricarboxyaniline, equivalent, 1.5 equivalent, 3 equivalent, 4 equivalent is one time, 1.5 times, 3 times and 4 times of tricarboxyaniline.
[0049] ① 25mmol of tricarboxyaniline was added to the aqueous solution in which 3 equivalents of sodium hydroxide were dissolved.
[0050] ②Dissolve an equivalent amount of sodium nitrite in water, and then add this solution dropwise to the solution in ① at 0°C under stirring conditions. After completely dissolving, add 4 equivalents of 1mol / L hydrochloric acid solution and continue stirring for 2 Hour
[0051] ③Under the protection of argon, 1.5 equivalents of 3,5-dimethoxyaniline was added to the solution of ②, stirred and reacted in ice bath for 5 hours, and AZO monomer was obtained.
[0052] 2) Preparation of polymer composites containing azo side chains:
[0053] 20 mmol of 6-amino-hexanol was dissolved in concentrated hydrobromic acid (purity: 48%, that is, the mass p...
Embodiment 3
[0057] 1) The preparation of AZO monomer, based on the mole of tricarboxyaniline, equivalent, 1.5 equivalent, 3 equivalent, 4 equivalent is one time, 1.5 times, 3 times and 4 times of tricarboxyaniline.
[0058] ①30mmol of tricarboxyaniline was added to the aqueous solution in which 3 equivalents of sodium hydroxide were dissolved.
[0059] ②Dissolve an equivalent amount of sodium nitrite in water, and then add this solution dropwise to the solution in ① at 0°C under stirring conditions. After completely dissolving, add 4 equivalents of 1mol / L hydrochloric acid solution and continue stirring for 2 Hour
[0060] ③Under the protection of argon, 1.5 equivalents of 3,5-dimethoxyaniline was added to the solution of ②, stirred and reacted in ice bath for 5 hours, and AZO monomer was obtained.
[0061] 2) Preparation of polymer composites containing azo side chains:
[0062] 30 mmol of 6-amino-hexanol was dissolved in concentrated hydrobromic acid (purity: 48%, that is, the mass pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com