Potassium-doped carbon nitride composite bismuth oxybromide photocatalysis material and preparation method thereof
A photocatalytic material, potassium hydroxide technology, applied in the field of preparation of K-g-C3N4/BiOBr photocatalyst, can solve the problems of poor adsorption of pollutants, low utilization rate of solar energy, easy recombination of photogenerated carriers, etc., and achieve improved degradation efficiency , good performance, safe and simple operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
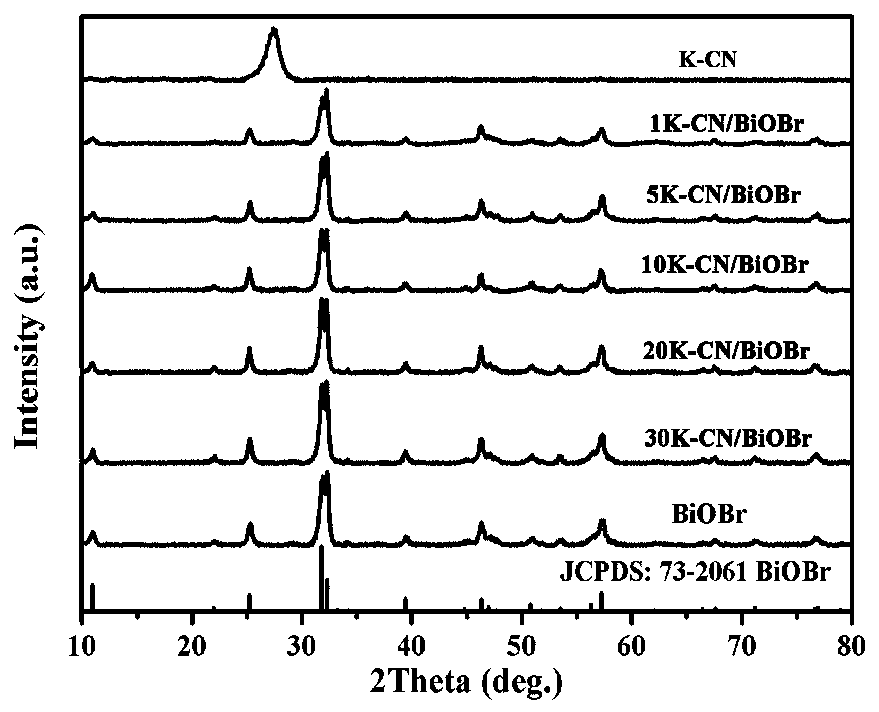
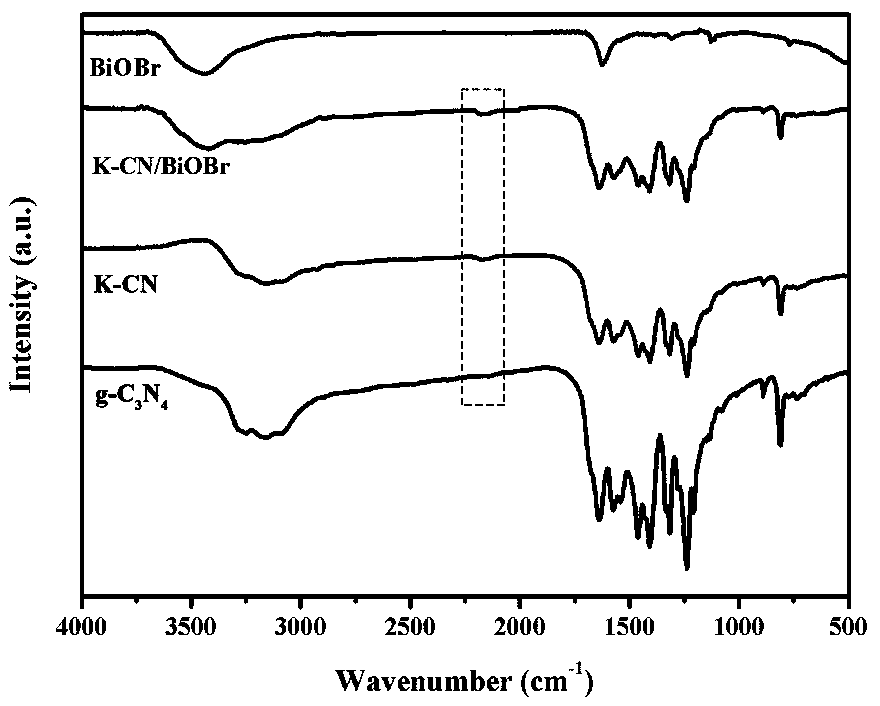
Image
Examples
Embodiment 1
[0027] (1) A certain amount of urea is ultrasonically dissolved in potassium hydroxide solution, and then the above mixed solution is dissolved at 80 o Dry in an oven at C;
[0028] (2) Calcining urea containing potassium hydroxide at a certain temperature to finally obtain yellow powder K-g-C 3 N 4 .
[0029] (3) Add K-g-C to deionized water 3 N 4 , and perform ultrasonic treatment to obtain a uniformly dispersed suspension;
[0030] (4) Add bismuth source and bromine source in the suspension of step (3), stir;
[0031] (5) Heat the suspension in step (4) in a water bath, wash and dry the reacted mixture to obtain 20K-g-C 3 N 4 / BiOBr photocatalytic material;
[0032] In the above-mentioned method, the potassium hydroxide solution concentration described in step (1) is 0.5 mol / L, and consumption is 10 mL, and the consumption of urea is 15 g.
[0033] In the above method, the calcining temperature described in step (2) is 550 o C, the calcination time is 4 h, the hea...
Embodiment 2
[0038] (1) A certain amount of urea is ultrasonically dissolved in potassium hydroxide solution, and then the above mixed solution is dissolved at 80 o Dry in an oven at C;
[0039] (2) Calcining urea containing potassium hydroxide at a certain temperature to finally obtain yellow powder K-g-C 3 N 4 .
[0040] (3) Add K-g-C to deionized water 3 N 4 , and perform ultrasonic treatment to obtain a uniformly dispersed suspension;
[0041] (4) Add bismuth source and bromine source in the suspension of step (3), stir;
[0042] (5) Heat the suspension in step (4) in a water bath, wash and dry the reacted mixture to obtain K-g-C 3 N 4 / BiOBr photocatalytic material;
[0043] In the above-mentioned method, the potassium hydroxide solution concentration described in step (1) is 0.5 mol / L, and consumption is 10 mL, and the consumption of urea is 15 g.
[0044] In the above method, the calcining temperature described in step (2) is 550 o C, the calcination time is 4 h, the heating...
Embodiment 3
[0049] (1) A certain amount of urea is ultrasonically dissolved in potassium hydroxide solution, and then the above mixed solution is dissolved at 80 o Dry in an oven at C;
[0050] (2) Calcining urea containing potassium hydroxide at a certain temperature to finally obtain yellow powder K-g-C 3 N 4 .
[0051] (3) Add K-g-C to deionized water 3 N 4 , and perform ultrasonic treatment to obtain a uniformly dispersed suspension;
[0052] (4) Add bismuth source and bromine source in the suspension of step (3), stir;
[0053] (5) Heat the suspension in step (4) in a water bath, wash and dry the reacted mixture to obtain K-g-C 3 N 4 / BiOBr photocatalytic material;
[0054] In the above-mentioned method, the potassium hydroxide solution concentration described in step (1) is 0.5 mol / L, and consumption is 10 mL, and the consumption of urea is 15 g.
[0055] In the above method, the calcining temperature described in step (2) is 550 o C, the calcination time is 4 h, the heati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com