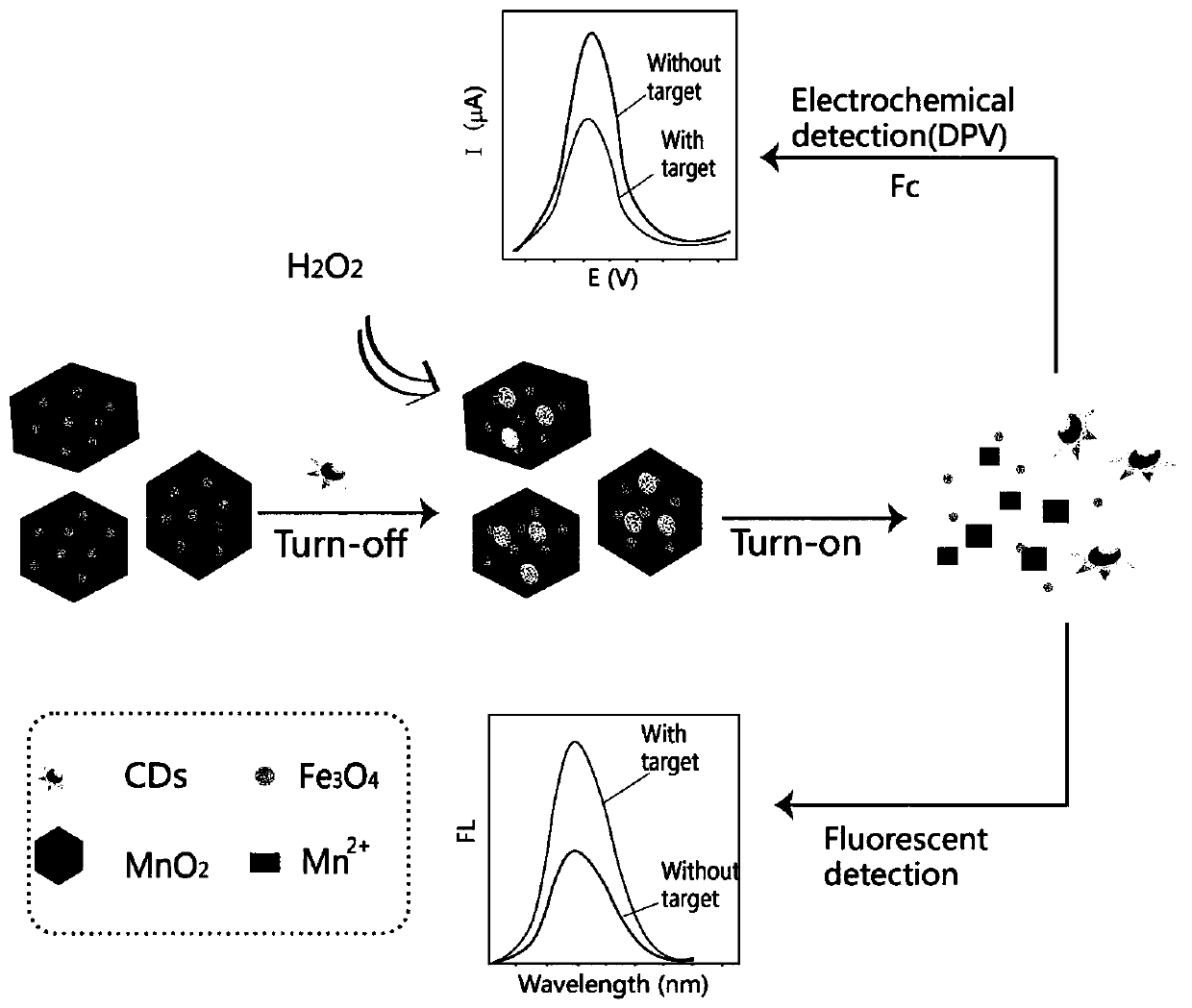
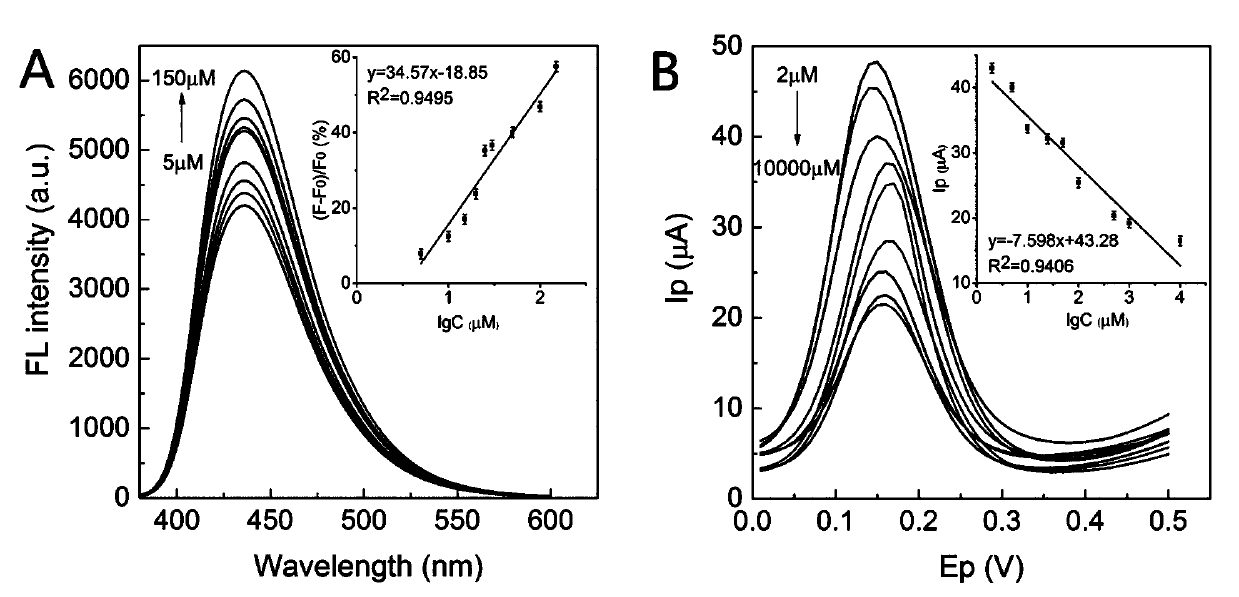
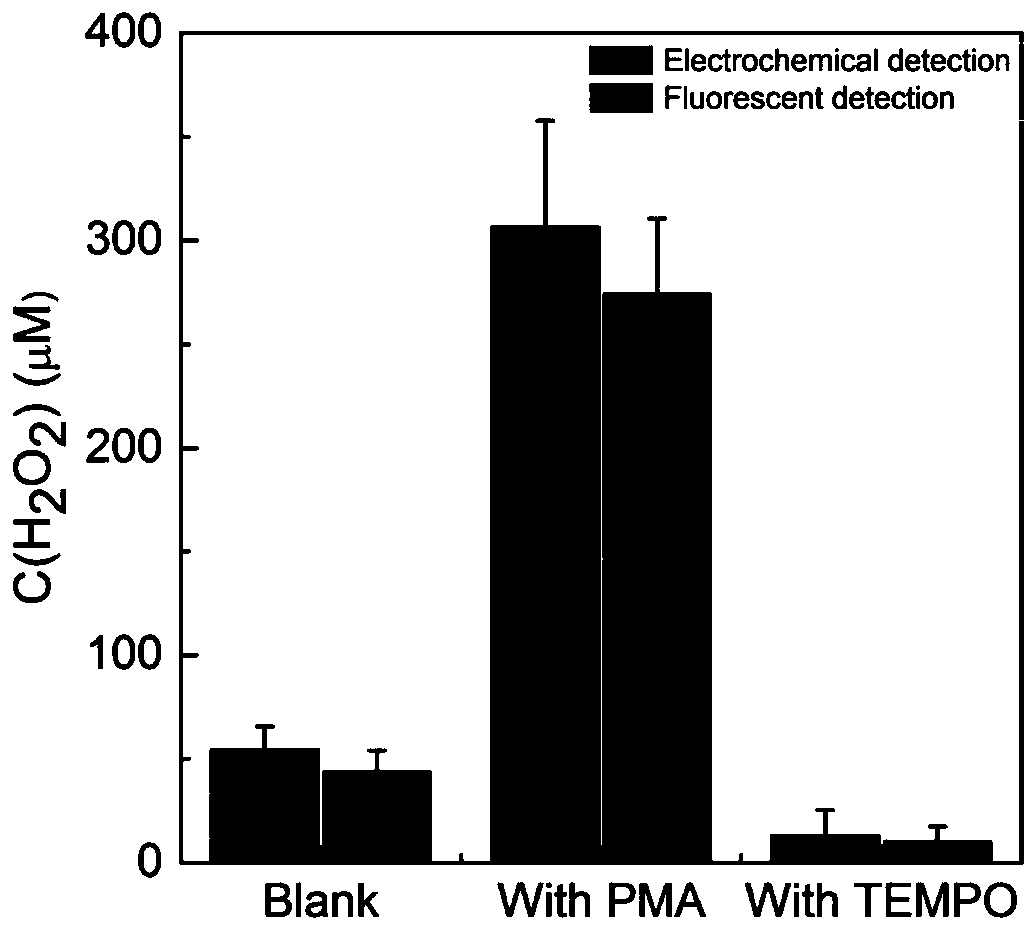
Method for detecting hydrogen peroxide by electrochemical and fluorescent double-signal sensor based on Fe3O4@MnO2 and carbon dots
A hydrogen peroxide and electrochemical technology, which is applied in the field of analysis and detection, can solve the problems of inaccurate determination of concentration, etc., and achieve the effects of simple and fast detection process, sensitive detection, and simple and fast preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] a) Fe 3 o 4 @MnO 2 Synthesis of: 0.42M Fe in 50 μL3 o 4 The solution was dispersed in 14mL water, and 3mgKMnO was added 4 Ultrasound to mix it evenly, add CTAB while stirring to emulsify the solution, then add 1.5mL MES buffer, react for 6h, to obtain a brown opaque liquid, magnetic separation to obtain a brown product that is Fe 3 o 4 @MnO 2 , washed 3 times with water, dried for later use.
[0041] b) Synthesis of carbon dots (CDs): Add 1 g of citric acid and 350 μL of diethylenetriamine to 10 mL of water, mix them evenly by ultrasonication, pour the liquid into a hydrothermal reaction kettle, raise the temperature to 200 ° C, react for 5 h, and then cool to After vacuum drying at room temperature, a brown crystalline solid, CDs, was obtained, which was ground into a powder with a mortar for later use.
[0042] c) Construction and conditions of the electrochemical sensor: at room temperature, mix the glassy carbon electrode with 2mL of the solution to be tested...
Embodiment 2
[0045] a) Fe 3 o 4 @MnO 2 Synthesis of: 0.42M Fe in 50 μL 3 o 4 The solution was dispersed in 14mL water, and 4mg KMnO was added 4 Ultrasound to mix it evenly, add CTAB while stirring to emulsify the solution, then add 2mL MES buffer, react for 7h, to obtain a brown opaque liquid, magnetic separation to obtain a brown product is Fe 3 o 4 @MnO 2 , washed 3 times with water, dried for later use.
[0046] b) Synthesis of carbon dots (CDs): Add 1 g of citric acid and 350 μL of diethylenetriamine to 10 mL of water, mix them evenly by ultrasonication, pour the liquid into a hydrothermal reaction kettle, raise the temperature to 220 ° C, and cool to After vacuum drying at room temperature, a brown crystalline solid, CDs, was obtained, which was ground into a powder with a mortar for later use.
[0047] c) The construction and conditions of the electrochemical sensor: at room temperature, the glassy carbon electrode was mixed with 2mL of the solution to be tested (containing 0...
Embodiment 3
[0050] a) Fe 3 o 4 @MnO 2 Synthesis of: 0.42M Fe in 50 μL 3 o 4 The solution was dispersed in 14mL water, and 5mgKMnO was added 4 Ultrasound to mix it evenly, add CTAB while stirring to emulsify the solution, then add 2mL MES buffer, react for 6h, to obtain a brown opaque liquid, magnetic separation to obtain a brown product is Fe 3 o 4 @MnO 2 , washed 3 times with water, dried for later use.
[0051] b) Synthesis of carbon dots (CDs): add 1 g of citric acid and 350 μL of diethylenetriamine to 10 mL of water, mix them evenly by ultrasonication, pour the liquid into a hydrothermal reaction kettle, heat up to 200 ° C, and cool to After vacuum drying at room temperature, a brown crystalline solid, CDs, was obtained, which was ground into a powder with a mortar for later use.
[0052] c) The construction and conditions of the electrochemical sensor: at room temperature, the glassy carbon electrode was mixed with 2mL of the solution to be tested (containing 0.1MKCl, 1mMFc, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com