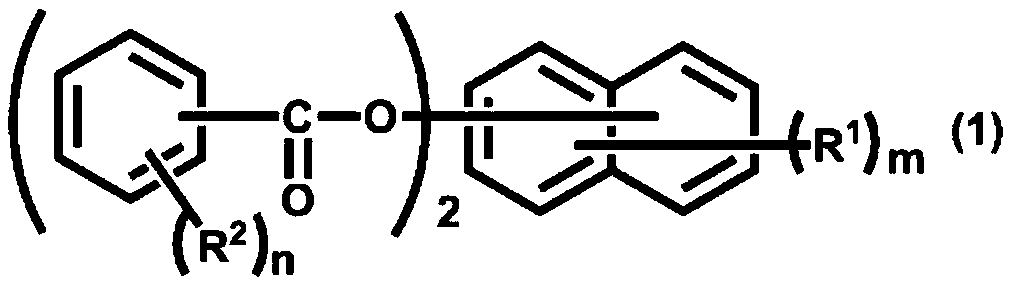
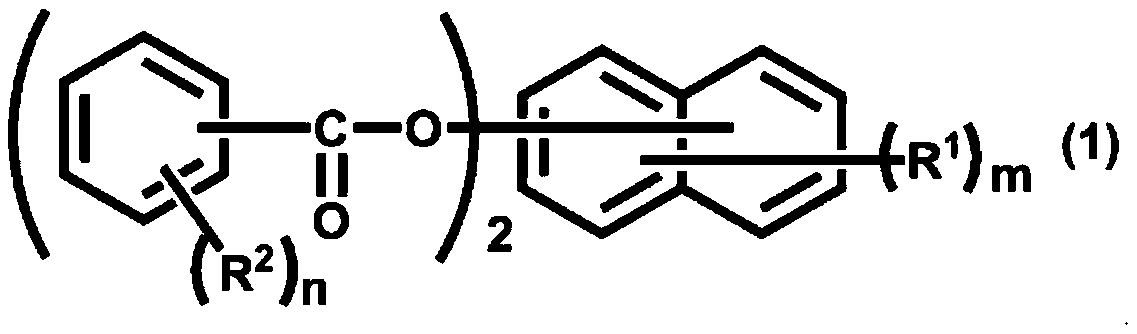
Active ester compound and curable composition
A curable composition and compound technology, applied in organic chemistry, electrical solid devices, semiconductor/solid device components, etc., can solve problems such as limited use, unsatisfactory elastic modulus, and high melt viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
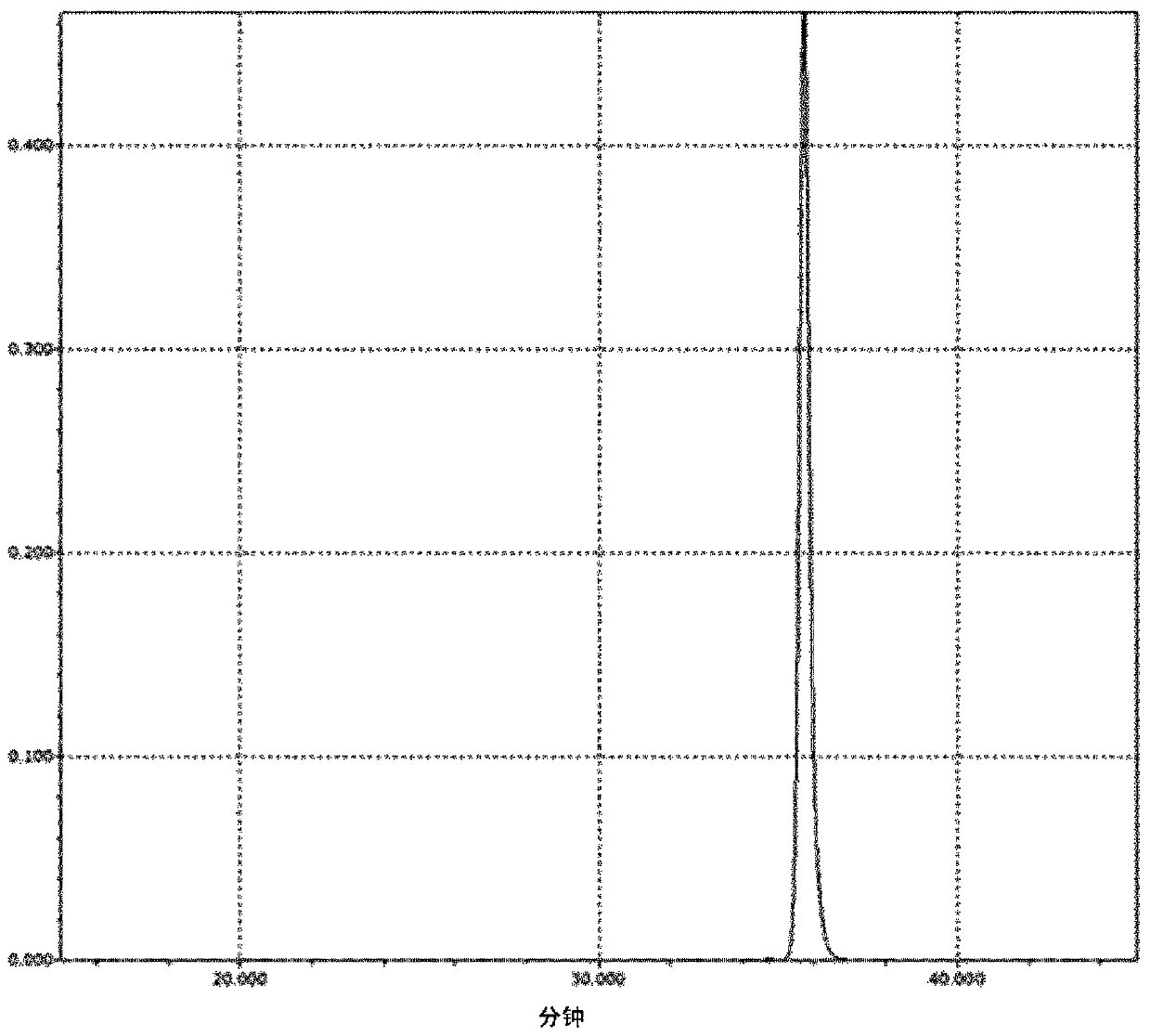
[0050] The manufacture of embodiment 1 active ester compound (1)
[0051] 160 g of 1,6-dihydroxynaphthalene and 1,100 g of toluene were put into a flask equipped with a thermometer, dropping funnel, condenser, fractionating tube, and stirrer, and dissolved while replacing the system with nitrogen under reduced pressure. Next, 218 g of benzoyl chloride was charged and dissolved while substituting nitrogen under reduced pressure in the system. 0.6 g of tetrabutylammonium bromide was added, and 420 g of 20% aqueous sodium hydroxide solution was added dropwise over 3 hours while controlling the inside of the system to 60° C. or lower while purging nitrogen gas. After completion of the dropwise addition, stirring was continued for 1 hour to make it react. After the reaction was completed, the reaction mixture was left to stand for liquid separation, and the water layer was removed. After adding water to the remaining organic layer and stirring for about 15 minutes, the mixture wa...
Embodiment 2
[0052] The manufacture of embodiment 2 active ester compound (2)
[0053] 160 g of 2,7-dihydroxynaphthalene and 1100 g of toluene were put into a flask equipped with a thermometer, a dropping funnel, a condenser tube, a fractionation tube, and a stirrer, and dissolved while replacing the system with nitrogen under reduced pressure. Next, 218 g of benzoyl chloride was charged and dissolved while substituting nitrogen under reduced pressure in the system. 0.6 g of tetrabutylammonium bromide was added, and 420 g of 20% aqueous sodium hydroxide solution was added dropwise over 3 hours while controlling the inside of the system to 60° C. or lower while purging nitrogen gas. After completion of the dropwise addition, stirring was continued for 1 hour to make it react. After the reaction was completed, the reaction mixture was left to stand for liquid separation, and the water layer was removed. After adding water to the remaining organic layer and stirring for about 15 minutes, th...
Embodiment 3、4 and comparative example 1
[0057] Each component was mixed in the ratio shown in the following Table 1, and the curable composition was manufactured. The curable composition was poured into the template, and molded at a temperature of 175° C. for 10 minutes using a press machine. The molded product was taken out from the template, and cured at a temperature of 175° C. for 5 hours to obtain a cured product. The cured product was subjected to an evaluation test according to the procedure described below. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melt viscosity | aaaaa | aaaaa |
| Melt viscosity | aaaaa | aaaaa |
| Melt viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com