Insulin oral spray and preparation process method thereof
A technology for oral sprays and insulin, which is applied in aerosol delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of large particle size and long time of soybean lecithin, and achieve easy absorption and good preparation The effect of high particle size and light transmittance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The process prescription is as follows:
[0036]
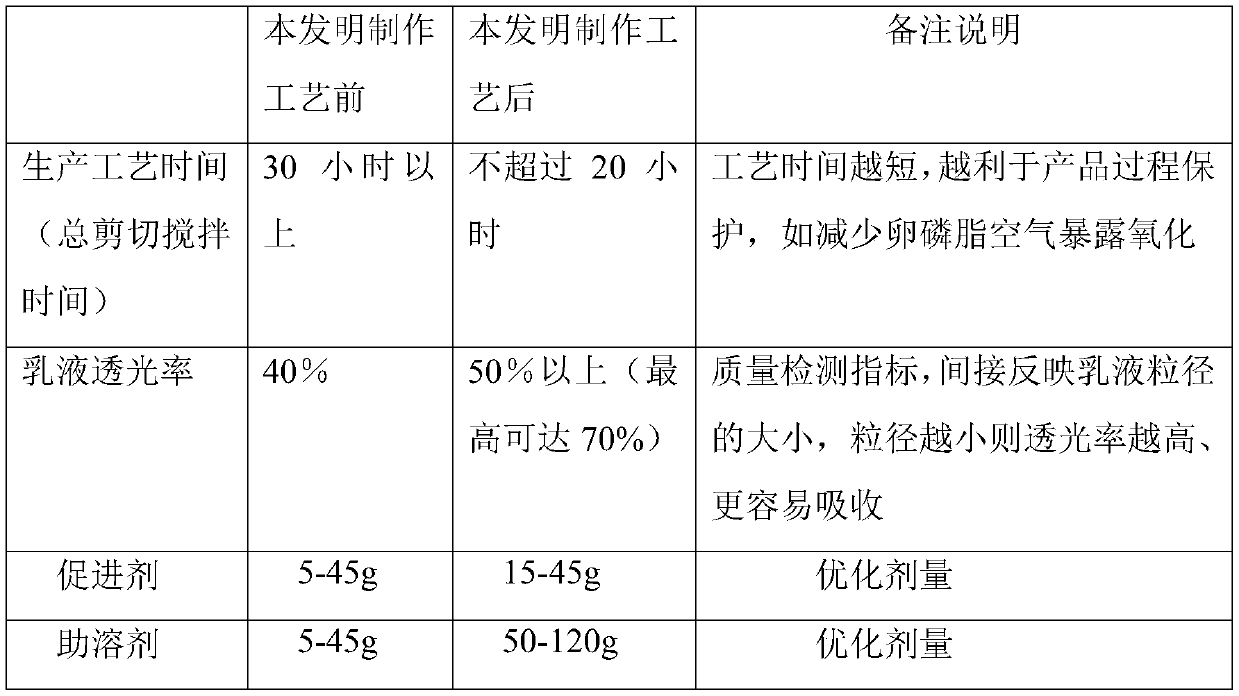
[0037] 25g of soybean lecithin and 50g of propylene glycol were sheared for 135 minutes, and the rotational speed of the shearing operation was maintained at 2000rpm to 3000rpm. After the shearing treatment, the light transmittance was above 40%; then the ethanol solution of borneol was added and stirred;
[0038]Then the above solution is added to the phosphate buffer solution, fully sheared, and the time is 610min. The rotating speed of the shearing operation is maintained at 2000rpm~3000rpm, and the light transmittance is 54.8% dilute solution, which is much better than that of the prior art polypeptide The light transmittance obtained by the drug oral spray is about 40% with a dilute solution.
Embodiment 2
[0040] The process prescription is as follows:
[0041]
[0042] 25g of soybean lecithin and 75g of propylene glycol were sheared for 113 minutes. The rotational speed of the shearing operation was maintained at 2000rpm-3000rpm. After the shearing treatment, the light transmittance was above 40%. Add the ethanol solution of borneol and stir;
[0043] Then the above solution is added to the phosphate buffer solution, fully sheared, and the time is 560min, and the rotating speed of the shearing operation is maintained at 2000rpm~3000rpm, and the light transmittance is 60.8% dilute solution, which is also much better than that of the prior art polypeptide The light transmittance obtained by the drug-like oral spray is about 40% with a dilute solution.
Embodiment 3
[0045] The process prescription is as follows:
[0046]
[0047] 25g of soybean lecithin and 120g of propylene glycol were sheared for 128 minutes. The rotational speed of the shearing operation was maintained at 2000rpm to 3000rpm. After the shearing treatment, the light transmittance was above 40%. Add the ethanol solution of borneol and stir until the soybean lecithin was completely dissolved. ;
[0048] Then add the above solution into the phosphate buffer solution, fully shear, it takes 650min, and the rotating speed of the shearing operation is maintained at 2000rpm~3000rpm, and the light transmittance is 52.2% dilute solution, which is also much better than that of polypeptides in the prior art The light transmittance obtained by the drug-like oral spray is about 40% with a dilute solution.
[0049] The above-mentioned examples only take recombinant human insulin as an example. The formulation of the insulin oral spray composition and its production process in the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com