A kind of synthetic method of α-acetyl-γ-butyrolactone
A synthesis method and technology of butyrolactone, applied in the direction of organic chemistry, etc., can solve problems such as hidden dangers, difficult to implement, easy to agglomerate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The first reaction kettle is a storage tank, and the storage tank is replaced with nitrogen. In a fully dry storage tank (20L) with a thermometer and a heat-conducting oil jacket, put 3.4kg of sodium metal into it under a nitrogen atmosphere, turn on the heat-conducting oil for heating, and when the temperature reaches 130°C, keep it warm for 2 hours to make the metal All the sodium is melted to obtain liquid metallic sodium in a molten state.
[0034] The second reactor is a condensation reactor. Add 16kg of γ-butyrolactone and 49.5kg of ethyl acetate into a fully dried condensation reaction kettle (100L) equipped with an electric stirrer, a condenser, and a thermometer, stir, and then slowly heat up to raise the temperature. When the system is refluxed, open the The dripping valve of the storage tank, according to the dropping rate of 2.5kg / h, drips the liquid metal sodium in the molten state into the condensation reactor. As the dripping proceeds, the temperature in...
Embodiment 2-32
[0037] Examples 2-32: α-acetyl-γ-butyrolactone was synthesized according to the method in Example 1 using the raw materials and parameters of each example in Table 1.
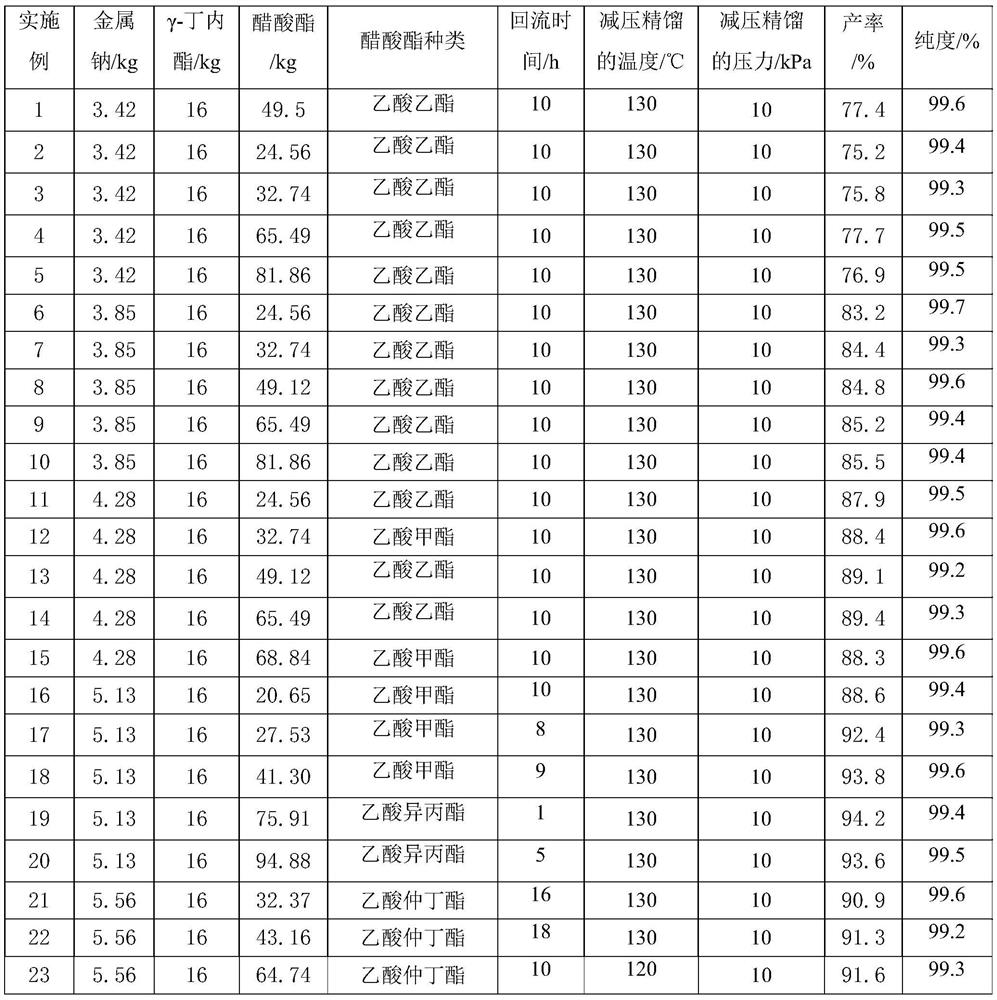
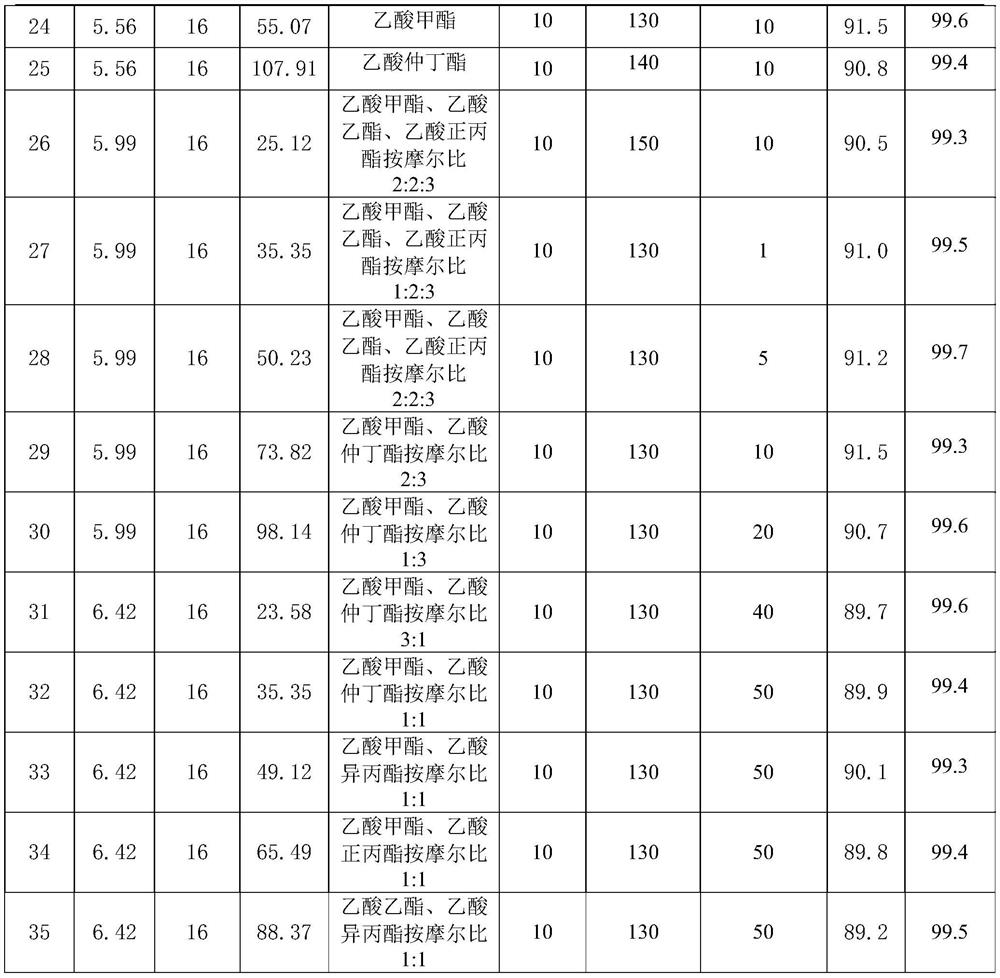
[0038] Raw material parameter and result in the embodiment 1-32 of table 1
[0039]
[0040]
[0041] In summary, the synthesis method of α-acetyl-γ-butyrolactone of the present invention does not need to add benzene as a reaction solvent or extractant, and is not only safe but also has high purity and high yield, with a purity greater than 99.0% and a yield greater than 87.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com